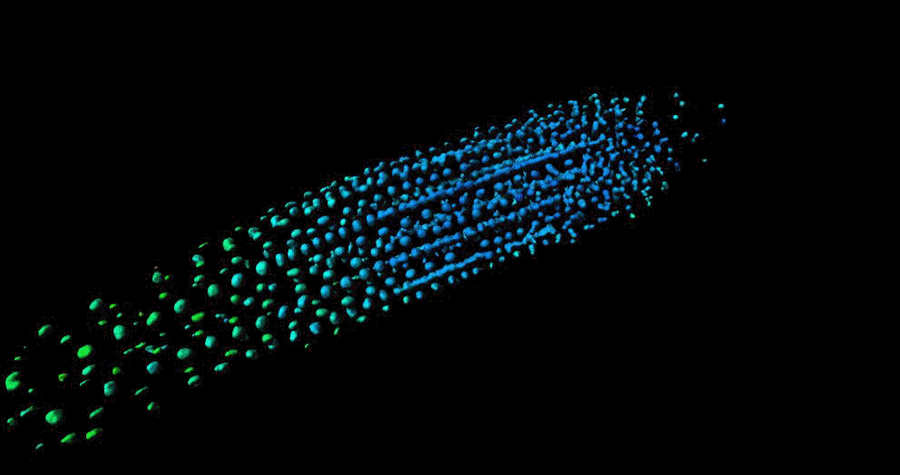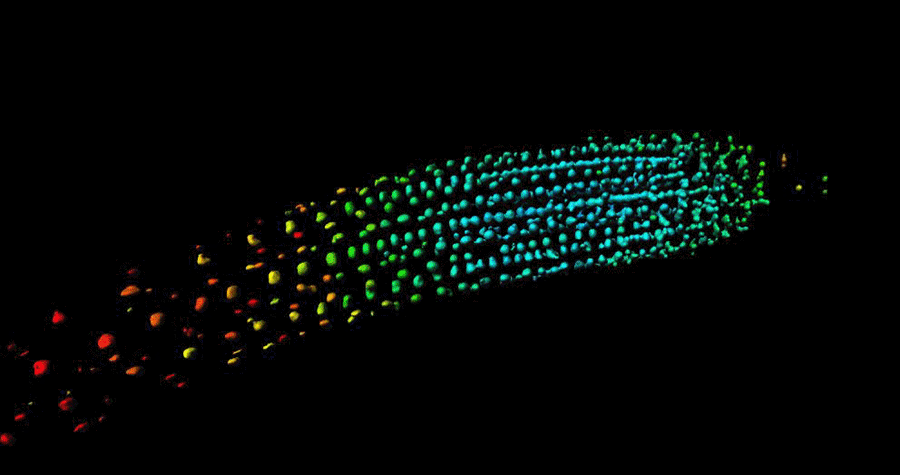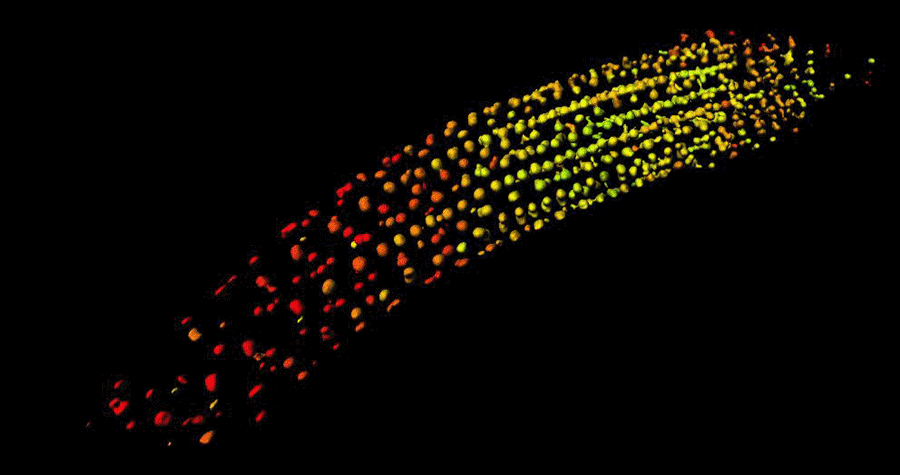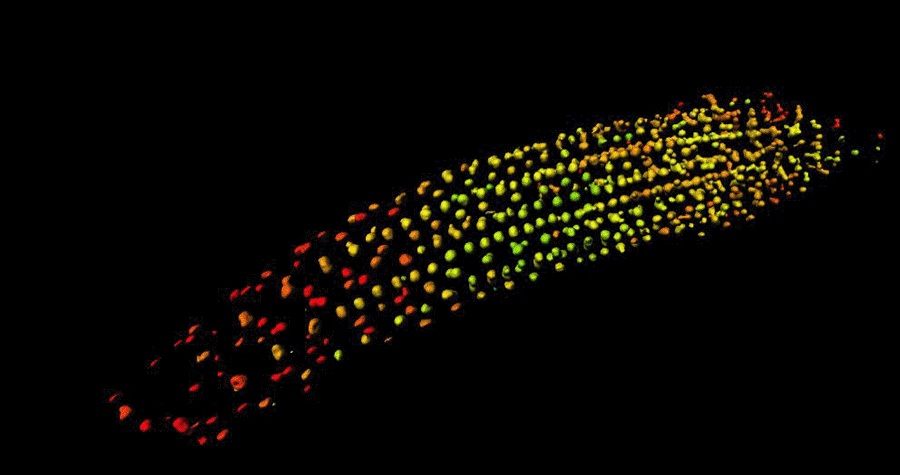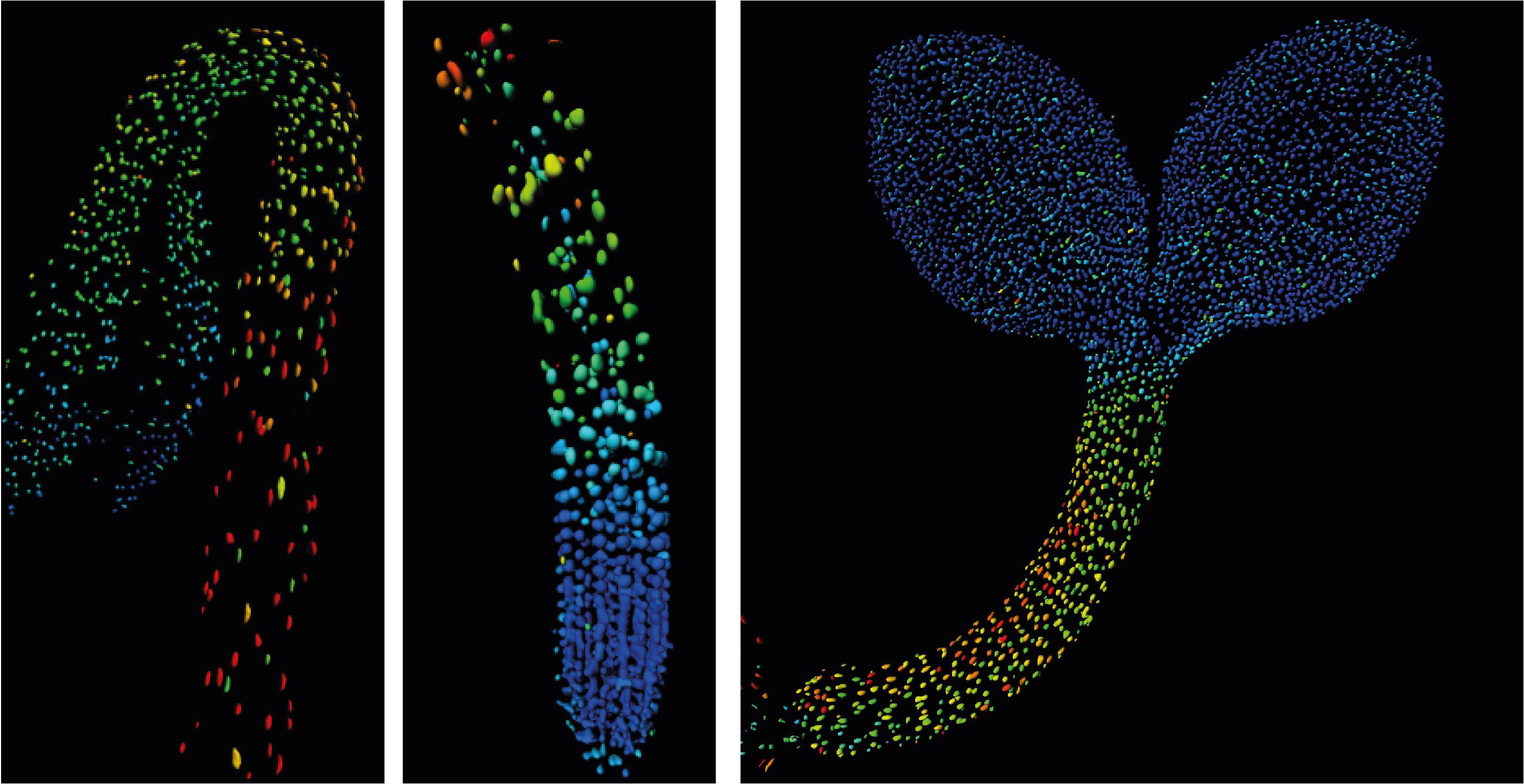
Biosensor imaging of Arabidopsis seedlings, measuring how the concentrations of the plant hormone gibberellin change as the plant grows. Images by Annalisa Rizza.
Regulating Gibberellin (GA)
In vivo gibberellin gradients visualised in rapidly elongating tissues
Developmental transcription factors, light, temperature, and other hormones all influence the concentration of the plant hormone gibberellin (GA), which promotes elongation growth in many plant tissues leading variously to altered stature, germination, fruiting, and crop yield. Understanding where and how GA is distributed is the first step to understanding how GA signalling leads to such a diverse range of responses.
The Jones Group has developed a fluorescent biosensor that permits the tracking of gibberellins at the cellular level in living plants. Team members, Dr Annalisa Rizza and Dr Ankit Walia, pursued the first lines of experimentation with the new biosensor, termed Gibberellin Perception Sensor 1 (GPS1) and found that gibberellins accumulate in rapidly elongating cell types of root tips, hypocotyls, and stamen filaments.
Hormone gradients visualised using a new fluorescent biosensorThe invention of a fluorescent biosensor that permits the tracking of gibberellins at the cellular level in living plants was developed through a collaboration between the Jones team and Wolf Frommer, formerly of Carnegie Plant Biology, Stanford. The Gibberellin Perception Sensor 1 (GPS1) allows the examination of hormone dynamics and growth at the cellular level during responses to their environment. |
The Jones Group previously characterised a positive correlation between levels of GA and cell length in roots (Rizza et al., 2017) and then determined both the key mechanisms controlling this cellular GA pattern and much of the quantitative relationship between the pattern and root cell elongation (Rizza et al., 2021).
The team then focused on revealing key GA enzyme expression patterns in hypocotyls and the transcription factors controlling them.
It has long been known that darkness elevates GA in hypocotyls, thus leading to dramatic elongation growth of etiolated seedlings. With nlsGPS1 imaging the Jones Group was able to bring into focus a cellular map of this phenomenon in wild-type and light signalling mutants. Interestingly, in certain mutants the spatial correlation between cell length and GA levels is broken.
In plants expressing GPS1, we observe gradients of GA in elongating root and shoot tissues. We now aim to understand how a series of independently tuneable enzymatic and transport activities combine to articulate the GA gradients that we observe. We further aim to discover the mechanisms by which endogenous and environmental signals, for example the light environment, regulate these GA enzymes and transporters.
Integrating maths and plant science to explain how plant roots generate a hormone gradientThe Jones Team, which developed a biosensor that first recorded that a distinct gradient of the plant growth hormone gibberellin correlated with plant cell size, has now revealed how this distribution pattern is created in roots.
|