Axillary buds are located at the base of each leaf. Initially dormant, each can grow into a branch. To study how branching is regulated by local signalling within each bud and by systemic signalling from other buds, we used stem sections with two axillary buds and their associated leaves (left). This signalling network influences, for example, whether one bud grows and rapidly inhibits the other (middle), or whether both buds grow simultaneously (right). Images by Zoe Nahas.
From bud to branch: How buds communicate to shape plant architecture
New model reveals how local and systemic signals combine to regulate shoot branching.
Scientists have shown that two previously separate modes of plant branching regulation can be connected, revealing the most complete picture yet of how plants control their architecture.
Published in PLOS Biology, researchers from the Sainsbury Laboratory Cambridge University (SLCU) demonstrate how local signals in plant buds link up with whole-plant hormone flows to control branching, with implications for future crop improvement.
At the base of every leaf is a tiny cluster of stem cells, known as an axillary meristem, with the potential to grow into a new branch. Whether these meristems remain dormant or start to grow depends on a mix of environmental signals, familiar to all good gardeners who know that fertiliser and light can affect how bushy their plants become.
Gardeners also know that they can shape shoot architecture by pruning – removing dominant growing shoots to release dormant buds in the base of leaves further down the shoot.
Researchers have long been interested in how all these signals are co-ordinated. One coordination hub is the transcription factor BRANCHED1 (BRC1), which acts locally within individual buds to inhibit their growth. Its activity is regulated by hormones that relay nutrient signals from the root, and by local light quality.
This new research suggests that BRC1 contributes to systemic shoot branching regulation by altering the export of the plant hormone auxin from the bud into the main stem.
Auxin in the main stem has been known to regulate bud activity for almost a century. More recently this has been attributed at least in part to its ability to prevent auxin export from buds, which appears to be necessary for their growth.
In this way, buds effectively compete to export their auxin into the stem and this can account for why removing active buds allows dormant buds to grow.
The new research combines mathematical modelling with bud growth measurements, providing evidence that local differences in BRC1 expression between buds helps determine which buds are more competitive, while systemic auxin transport properties set the total number of branches a plant can sustain.
This creates a flow of information across the plant that each bud interprets locally, allowing the plant to regulate when and where branches form.
The result is a unified model that explains how plants tune the number and the position of branches to integrate the overall balance of growth across the shoot with environmental conditions such as light or nutrient availability.
For example, a bud in a favourable position, such as in better light, may express less BRC1 and so grow more quickly, while a neighbouring bud with higher BRC1 expression may remain dormant. At the same time, systemic regulation through auxin transport ensures that the overall branching load is kept in balance across the whole plant.
Dr Zoe Nahas, the study’s first author, said: “Our main finding is that by modulating auxin transport, local BRC1 expression in each bud could contribute to the systemic control of branching.
“What’s surprising is that a very simple mathematical model of the interaction between two buds, where each bud is represented as promoting its own growth and inhibiting the growth of the other, can capture so many of the branching behaviours we actually see in the lab.”
Fig 1. 2-node explants capture key properties of bud regulation (originally published in Nahas et al, (2025) PLOS Biology)
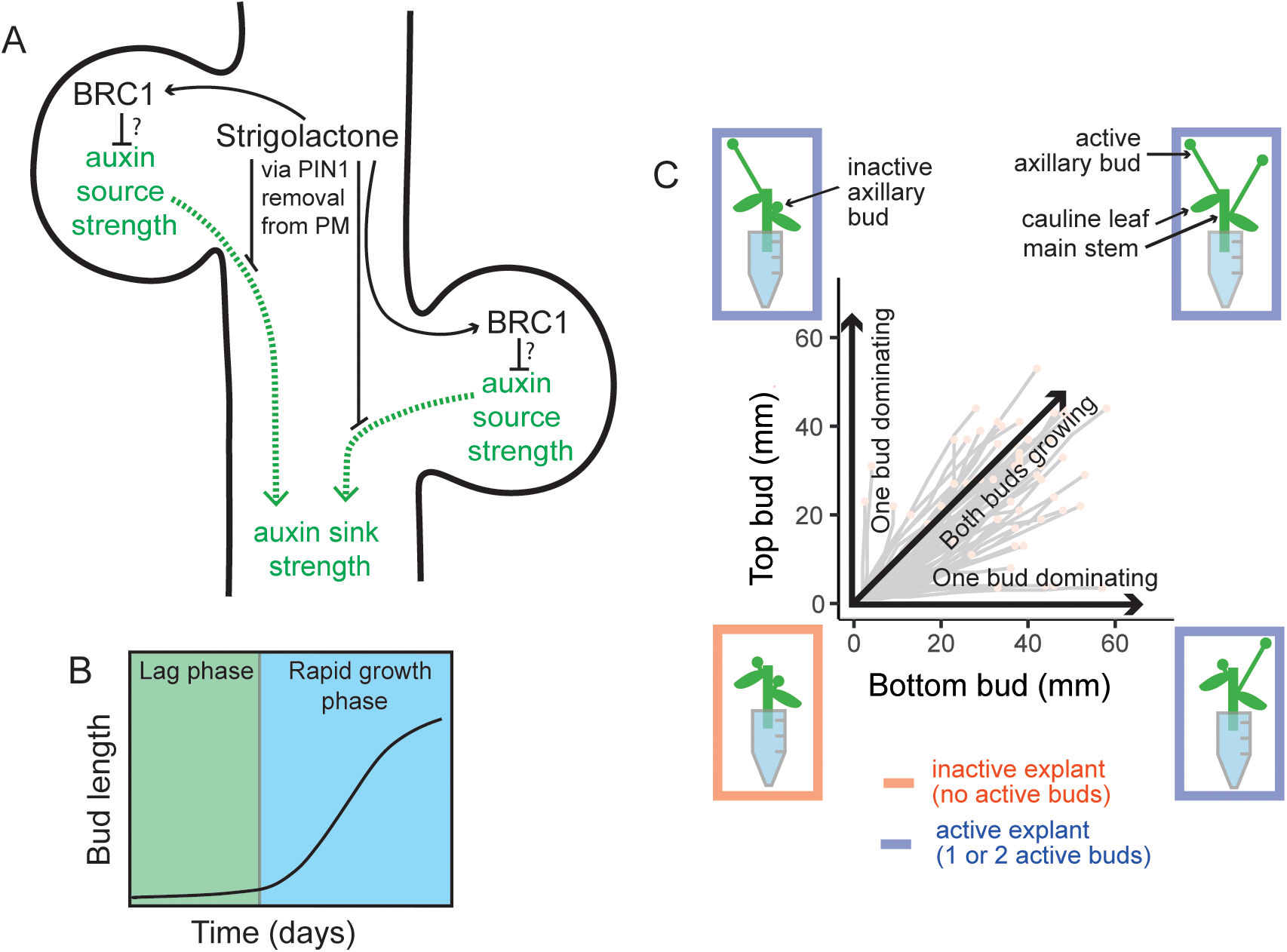
(A) A stem segment with two axillary buds illustrates two regulatory hubs controlling shoot branching (i) local expression of the transcription factor BRC1, a repressor of bud activation, and (ii) systemic regulation of the auxin transport network. A canalization-based model of shoot branching postulates that bud activation requires the establishment of canalized auxin transport from the bud into the main stem, the dynamics of which is influenced by autocatalytic feedback in auxin flow between the bud and the stem, and the relative auxin source and sink strengths of the bud and stem, respectively. The relationship between BRC1- and auxin-transport-mediated regulation is not known. (B) Arabidopsis bud activation occurs in at least two phases: a slow-growing lag phase, then a switch to rapid outgrowth. Typical timescale 10–12 days. Modified from Nahas and colleagues [46]. (C) Diagram illustrating the four possible growth outcomes for bud activation on 2-node explants, and their representation in a Mitchison plot. Mitchison plots present the length of the top bud versus that of the bottom bud over time in each explant. Explants where at least one bud grows are termed active, otherwise, they are inactive. Within active explants, there are three possible outcomes: both buds grow, or only either the top or the bottom bud activates. All graphics were drawn by hand using Adobe Illustrator by Zoe Nahas.
The model not only predicted experimental outcomes under different genetic and hormonal conditions, but also could accommodate data about a previously unknown region of the PIN1 auxin transporter that mediates its response to the hormone strigolactone, offering new molecular insight into how hormones influence bud growth.
This new fundamental understanding of how local and systemic signals are integrated in plant branching could help scientists design new strategies to optimise crop yield, resilience, and resource use.
Professor Ottoline Leyser, senior co-lead of the research, said: “Plants have extraordinary flexibility in their growth, and branching is a key part of this adaptability. The unified model we have developed will help us to understand how plants integrate multiple sources of information to determine where to invest in growth.”
Professor James Locke, senior co-lead, added: “This work brings together experiments and modelling to show how local and systemic signals can interact to control bud growth. What’s striking is that such a simple model can capture the range of branching behaviours we see experimentally.”
Reference
Zoe Nahas, Anthony John Bridgen, Torkel E. Loman, Jean Dillon, Katie Abley, Dora L. Cano-Ramirez, Fabrizio Ticchiarelli, Madeleine Seale, James C. W. Locke and Ottoline Leyser (2025) A BRC1-modulated switch in auxin efflux accounts for the competition between Arabidopsis axillary buds. PLOS Biology.
DOI: 10.1371/journal.pbio.3003395
Funding
This research was supported by the Gatsby Charitable Foundation and Biotechnology and Biological Sciences Research Council (BBSRC).