SLCU researchers have demonstrated that the circadian clock is more complex than a simple ‘on-off switch’ when it comes to controlling cell division.
The ability of a cell to replicate itself is what defines cells as living things. This elegant process where a parent cell divides into two daughter cells hides an incredibly complex series of biochemical reactions that scientists have been trying to understand since cell division was first observed in 1835. One of the core questions they are trying to answer is – how do cells decide when to divide? Size, time and external environment are known to have an influence, but how they interact with each other to affect a cell’s life-cycle is still not fully understood.
Researchers have taken a step closer to answering this question by demonstrating that the internal 24-hour biological clock (circadian clock) is more complex than a simple ‘on-off switch’ when it comes to controlling cell division.
Published in the journal Proceedings of the National Academy of Sciences, a new study by scientists at the Sainsbury Laboratory Cambridge University (SLCU) and the Imperial College London shows that the circadian clock continuously modulates cell division throughout the day and night in cyanobacteria. This finding rewrites the most recent understanding that the clock was acting only as an ‘on-off switch’ and provides an insight into how the cyanobacterial clock controls innate cell growth and division principles that organisms follow.
|
What is a circadian clock? A circadian clock is an organism’s internal timing device that schedules biological activities over a 24-hour cycle. It clock responds primarily to light and darkness. The clock has been shown to be connected to cell division in many systems, from unicellular organisms to mammals. For example, in mammals, a master clock in the brain coordinates the circadian clocks in the rest of the mammal’s body to control hormone production, cell regeneration, behaviour and other biological processes linked to this daily cycle. Plant circadian clock(s) regulate the timing of flower opening, leaf movement, photosynthesis and even fragrance emission. |
Cell cycle regulation
Actively dividing living cells pass through a series of growth and development stages called the ‘cell cycle’. Cells are ‘born’ by a division of a parent cell and ‘reproduce’ by division to make two new daughter cells. Models to explain cell size regulation have been developed over a number of years – the sizer rule (that cells divide when they reach a certain size), the timer rule (that cells divide after a certain period of time) and the adder rule (that cells divide after they have increased their birth size by a certain amount). Deciding when and at what size to divide is a critical decision for cells. If something goes wrong with the cell cycle, it can have a big impact, such as with cancer, which is a disease of uncontrolled cell division
|
Cyanobacteria are bacteria that obtain their energy through photosynthesis. Cyanobacterium Synechococcus elongatus is a common organism found in freshwater. It has one of the simplest circadian clocks in nature encoded by just three genes, which makes it ideal for studying how the circadian clock influences cell growth and gene regulation. |
Lead author of the research and member of James Locke’s group at SLCU, Dr Bruno Martins, examined how the 24-hour circadian clock and environmental cycles modulate cell size control and division timing in the cyanobacterium Synechococcus elongatus using single-cell time-lapse microscopy.
Dr Martins' time-lapse microscopy movie clearly shows cell growth is arrested by darkness during cycles of 12-hours light and 12-hours dark.
The team designed a set of experiments with colonies of cyanobacteria to pick apart the influence of time of day, size of cell, and the presence of light on cell division.
First, they observed how cell division rates under constant light conditions differed between cyanobacteria that possessed circadian clocks and cyanobacteria that had their circadian clock genes removed.
Using the division patterns from these experiments, and what was thought to influence them, they then designed models to predict what would happen if the light changed over the course of future experiments.
In the subsequent experiments, the team found that rather than the circadian clock acting like an on/off switch or ‘gate’, forbidding cell division at certain times, it acts to fine tune the process by decreasing division at certain times and accelerating it at others.
What they found matched the second set of experiments well, meaning their models successfully described the mechanisms at play.
“The prevalent idea is that cell division is freely ‘allowed’ at certain times of the day (gate open) and restricted at other times (gate closed),” Dr Martins said. “We demonstrated that the clock actually continuously modulates the probability of cell division throughout day and night, which impacts on the regulation of cell size. In fact, the clock generates two subpopulations of cells – a population of cells that are born earlier in the day and a population of cells that are born later in the day. These two subpopulations are distinctly sized and their cell cycle duration is distinct as well. Cells born in the early part of the day grow to a smaller size before dividing again, because they seem to be in a “rush” to divide before the end of the day. In contrast, cells born later in the day are in less of a “rush”, and therefore they grow to a bigger size, and avoid dividing in the period that normally corresponds to darkness at night.”
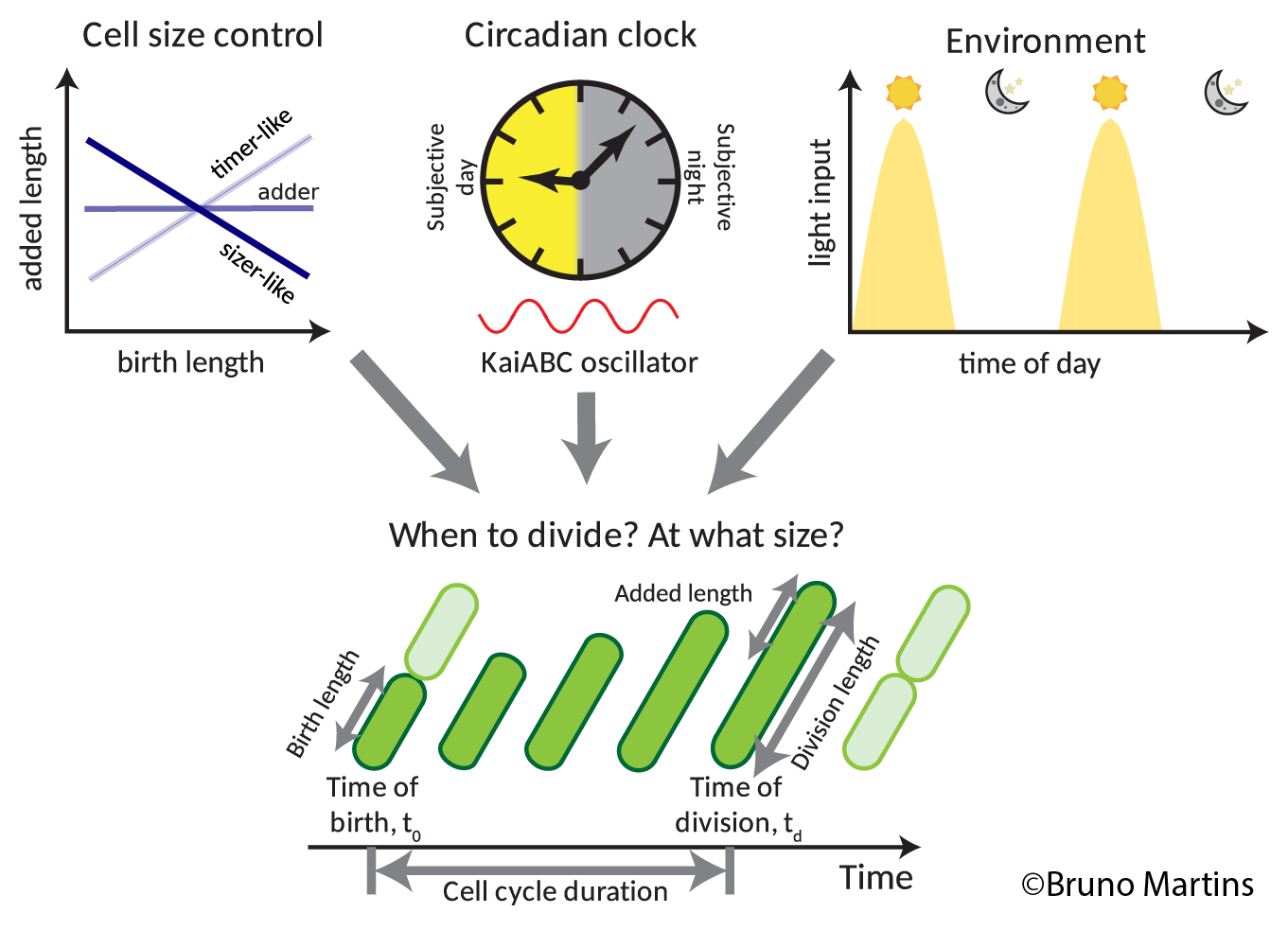
Dr Martins wanted to see whether the circadian clock was playing any role in telling cells when to divide and at what size to divide.
Continuous coupling with circadian clock
Dr Martins says that this mechanism arises from the continuous coupling of the clock to the cell cycle, which steers cells away from dividing at dawn or dusk. “Under natural light-dark cycles, cell division is restricted to when light levels are highest,” he said. “This makes good sense as the cyanobacterium does not have access to light at night-time to obtain energy, and so it might not be sensible to divide just before dark without a source of energy.”
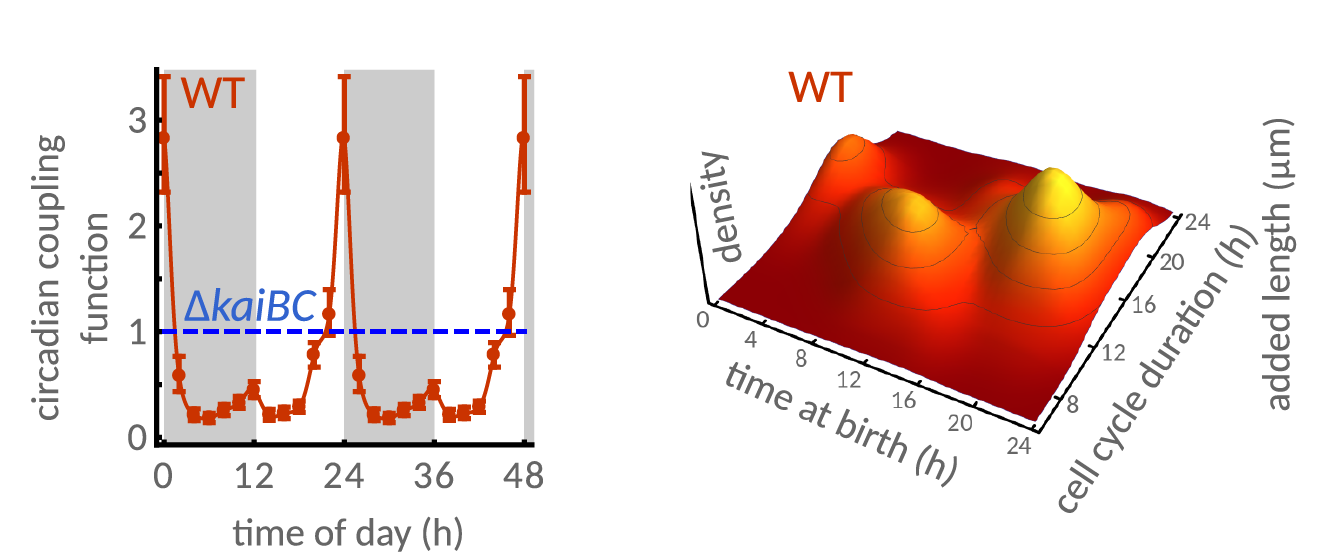
The circadian clock generates two subpopulations of cells.
Dr Martins joined up with Dr Philipp Thomas from the Department of Mathematics at Imperial College London and together they developed a model that explains how this two-population result emerges from the interaction of cell size control with the clock.
“This model is helping us to better understand and predict the effects of complex interactions between the environment, clock, and cell size control. Our next step is to look at what molecules and genes are involved in this process and to explore its evolutionary function, Dr Martins said.
Dr Thomas said: “Instead of acting as a strict gate for cell division, the circadian clock constantly influences the division rate throughout the day. Unpicking the complex interactions between cell size, clock and environment was only possible through the careful combination of experiments and iterative models that determined the contribution of the factors at play.”
This research was funded by OpenPlant, BBSRC, the Royal Commission for the Exhibition of 1851 and the Gatsby Charitable Foundation.
‘Cell size control driven by the circadian clock and environment in cyanobacteria’ by Bruno M. C. Martins, Amy K. Tooke, Philipp Thomas, and James C. W. Locke is published in Proceedings of the National Academy of Sciences.

 Cyanobacterium
Cyanobacterium



