An interdisciplinary research collaboration between SLCU and the University of Oxford has engineered a novel synthetic plant-microbe signalling pathway that could provide the foundation for transferring nitrogen fixation to cereals.
Published in Nature Communications today, the team of plant scientists, microbiologists and chemists used synthetic biology techniques to design and then engineer a molecular dialogue between plants and the bacteria surrounding their roots in a zone called the rhizosphere. This synthetic signalling system could be a vital step towards successfully engineering nitrogen-fixing symbiosis in non-legume crops like wheat and maize.
Professor Giles Oldroyd, who leads a SLCU research group studying the mechanisms involved in plants forming beneficial interactions with nitrogen fixing bacteria, said the synthetic signalling system was likely to provide a key component in further work to engineer nitrogen fixation into cereals: "The long-term aim of our research is to broaden the plant host species that can accommodate nitrogen-fixing bacteria, in particular in cereal crops. There is great potential to deliver more sustainable and secure food production systems through engineering nitrogen fixation in cereals, with particular potential to deliver significant yield improvements to the poorest farmers in the world. The results from this research means that we could potentially use this transkingdom signalling pathway to control root microbiota in the cereal rhizosphere for delivering many beneficial services and reducing fertilisers."
A key step in establishing symbiotic relationships between plants and microbes is to get the plant and bacteria communicating with each other through chemical signals, in this case using compounds called rhizopines.
Dr Ponraj Paramasivan, joint lead author from Professor Oldroyd's group said the team transferred rhizopine synthesis genes into barley to assess whether they could engineer rhizopine synthesis in cereals: “We confirmed the barley synthesised and then exuded rhizopine to its rhizosphere. We then measured the signalling between barley roots and rhizosphere bacteria and found a significant level of communication was occurring in most bacterial colonies.
“A key advantage of this synthetic signalling pathway is that only the specific crop plant that is engineered to produce the signal will benefit. This means that weeds that currently benefit just as much as the target crop from the application of chemical fertilisers, will not benefit from these synthetic plant-microbe associations as they do not produce this novel signalling molecule to communicate with bacteria.”
Joint lead author, Dr Barney Geddes from Professor Philip Poole’s lab at Oxford’s Department of Plant Sciences, said enhancing the root microbiota has enormous potential for improving crop yields in nutrient-poor soils and reducing chemical fertiliser use: “Plants influence the microbiota of their rhizosphere by sending out chemical signals that attract or suppress specific microbes. Engineering cereal plants to produce a signal to communicate with and control the bacteria on their roots could potentially enable them to take advantage of the growth-promoting services of those bacteria, including nitrogen fixation. To do this we selected a group of compounds normally produced by bacteria in legume nodules, called rhizopines. First we had to discover the natural biosynthetic pathway for rhizopine production, and then design a synthetic pathway that was more readily transferred to plants. We were able to transfer the synthetic signalling pathway to a number of plants, including cereals, and engineer a response by rhizosphere bacteria to rhizopine.”
Joint lead author Dr Amelie Joffrin in Professor Stuart Conway’s lab at Oxford developed a new stereoselective synthesis of key rhizopine: “The synthetic chemistry was essential to provide compounds that enabled the investigation of rhizopine biosynthesis and its transfer from bacteria to plant. In particular, the rhizopines produced allowed us to confirm which was the naturally active enantiomer (“hand”) of a key bioactive compound.”
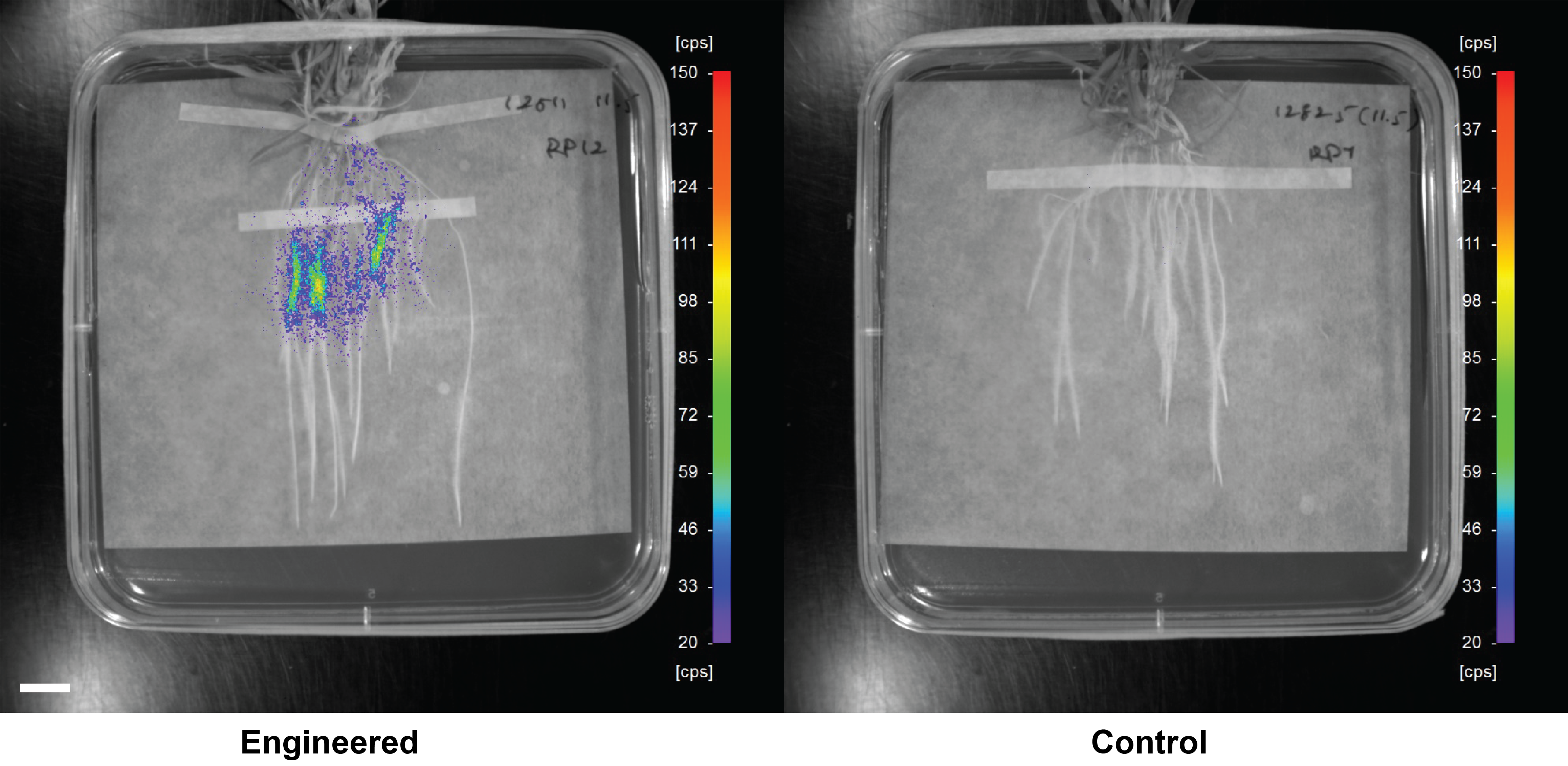
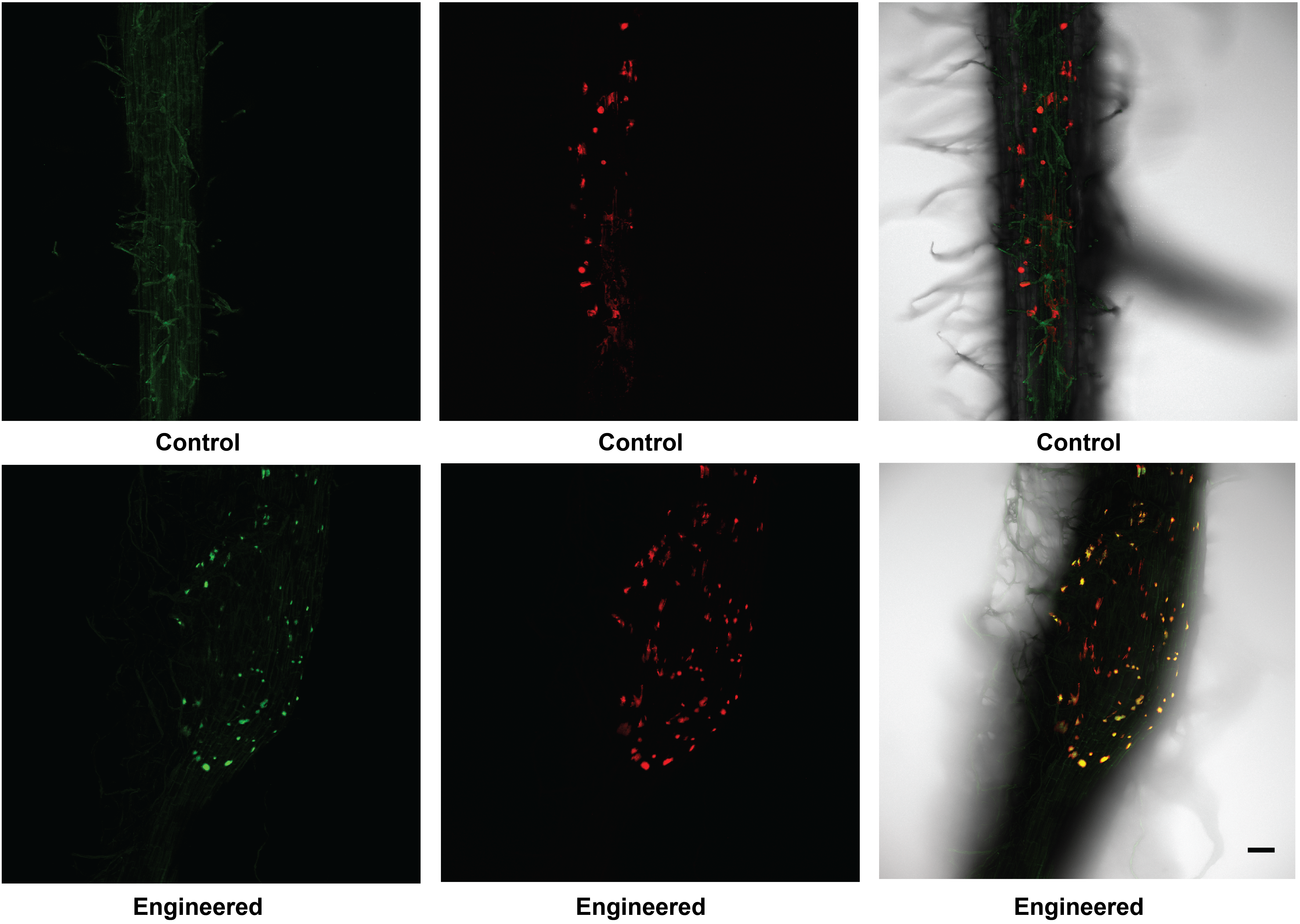
Future work in the Poole, Oldroyd and Conway laboratories will focus on how plants can control key processes in root bacteria such as nitrogen fixation, phosphate solubilisation and plant growth promotion. This opens up the world of the bacterial microbiome and its diverse metabolism to control by plants and in particular the cereals. It is likely to be a key component in attempts to engineer nitrogen fixation into cereals.
Funding
This research was supported through a joint grant from the BBSRC and NSF as well as the EPSRC.
Full paper
Barney Geddes, Ponraj Paramasivan, Amelie Joffrin, Amber Thompson, Kirsten Christensen, Beatriz Jorrin, Paul Brett, Stuart Conway, Giles Oldroyd, and Philip Poole. (2019) Engineering transkingdom signalling in plants to control gene expression in rhizosphere bacteria. Nature Communications. DOI: 10.1038/s41467-019-10882-x





