New research that has created the first three-dimensional (3D) computational model of a large mitotic spindle is providing crucial insights into how a plant cell divvies up genes when dividing in two.
The mitotic spindle is a molecular machine that helps separate chromosomes during cell division. Interactions among the component parts – largely microtubules of the cytoskeleton and additional regulatory proteins – form a dynamic structure within the cytoplasm of all eukaryotic cells (plants, animals and fungi). Despite its key role in cell division, the precise mechanisms by which the mitotic spindle organises and functions during cell division are not fully understood.
Scientists have taken a major step towards better understanding this molecular machine by creating a detailed 3D simulation of the spindle of Arabidopsis thaliana – the primary model plant used in plant science research around the world. Combining experimental and computer simulations they are revealing key molecular players that regulate its shape and function during cell division.
Published in Developmental Cell, this is possibly the most real-to-life simulation of a spindle to date, as only much smaller spindles such as the ones found in yeasts had been modelled in 3D before.
“This comprehensive model of spindle behaviour opens the door to future discoveries about the role of cell-cycle-dependent phosphorylation in plant cells. This study underlines the importance of computational modelling in advancing our understanding of cellular biology.”
ooter>Dr François Nédélec
“This comprehensive model of spindle behaviour opens the door to future discoveries about the role of cell-cycle-dependent phosphorylation in plant cells. This study underlines the importance of computational modelling in advancing our understanding of cellular biology.”
Unlike animal cells, which use a dedicated microtubule organiser called a centrosome, plants rely on other means to organise the two spindle poles of each daughter cell. Other elements such as the Augmin complex are conserved in plants and animals, and the present study points to augmin as a key regulator of spindle function.
The new model developed by an interdisciplinary research team led by the Department of Biology at the University of Hamburg and the Sainsbury Laboratory at the University of Cambridge, in collaboration with other researchers from Germany, England, France and Belgium, not only offers a better understanding of these processes but also provides a powerful framework for exploring how plants control cell division.
|
The mitotic spindle in plants For a cell to successfully divide after growth it needs to move its duplicated chromosomes into the right position so that an equal set of chromatids ends up in each daughter cell. In most animals and fungal cells, spindle microtubules nucleate from centrosomes or spindle pole bodies. Plant cells lack such structured microtubule organising centres and very little is known about spindle formation in plants or how they separate chromosomes into the resulting daughter cells. Many studies on cell division have used the frog Xenopus laevis or the baker's yeast Saccharomyces cerevisiae as model systems. However, the spindle in the frog is very large (40 µm) and contains approx. 300,000 microtubules. This size makes a computer simulation extremely difficult. In contrast, the spindle in yeast is relatively small (1.4 µm) and is formed by only about 40 microtubules. The spindle of Arabidopsis thaliana, a widely used model in molecular plant research, lies between these extremes with a length of approximately 8 µm and 2,000 microtubules. |
This new model highlights the role of CYCLIN-DEPENDENT KINASE B1 (CDKB1) and its partner CYCB3;1, which control spindle morphology through their interaction with the augmin complex.
The augmin complex, specifically its component ENDOSPERM DEFECTIVE1 (EDE1), was identified as a target of the CDKB1-CYCB3;1 complex. This interaction is crucial for maintaining proper spindle structure, as mutations in EDE1 lead to elongated spindles, similar to what is observed in CDKB1 and CYCB3;1 mutants.
These findings were further confirmed through the development of a dynamic 3D model using Cytosim, an open-source cytoskeleton simulation tool developed in the team of Dr François Nédélec. Dr Nédélec explained: “The spindle attaches to chromosomes through protein complexes called kinetochores, ensuring accurate chromosome alignment and segregation. This process is essential for maintaining the correct distribution of genetic material during mitosis. Using simulations in Cytosim, we were able to reduce the level of augmin, which resulted in spindle abnormalities, mirroring those seen in mutant plants.”
The simulation offers a detailed view of how different proteins interact during mitosis, including kinesins that move microtubules, and katanin, a protein that regulates spindle length by severing microtubules.
Dr Helen Saville and Claire Jacquerie worked on extending Cytosim to create the quantitatively-accurate spindle dynamic model in 3D in Arabidopsis roots. Dr Saville explained the modifications they made: “In addition to Brownian motion and filament bending elasticity, we added microtubule nucleation via three pathways —kinetochore, pole-induced, and augmin-mediated, each with specific properties and dynamic behaviors to match the plant root spindle.”
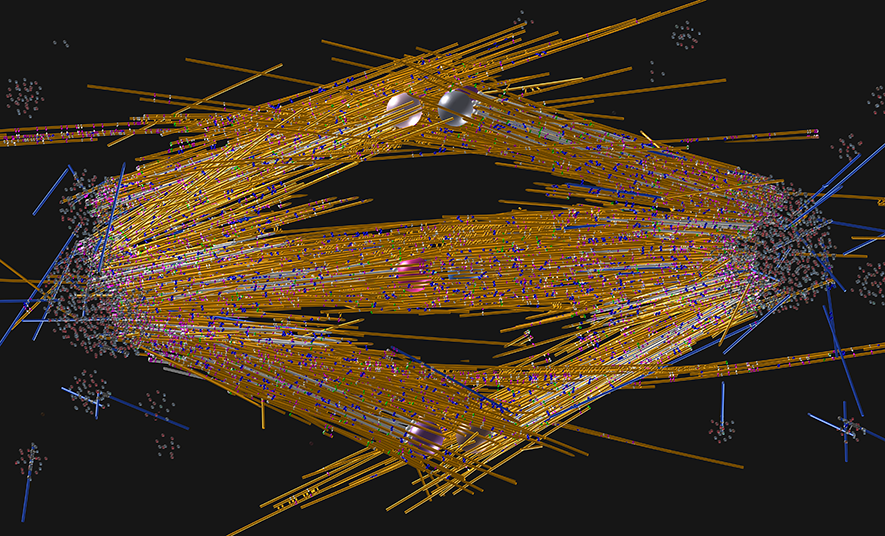
A model of the mitotic spindles found in plant roots. The chromosomes, represented here by their kinetochores (two spheres for each chromosome), reside in the middle of the picture. Around them, microtubules (yellow, white, and blue lines) organize into a strong mechanical scaffold. Microtubules, which are the main structural elements of the scaffold, are additionally connected by various molecular motors of the kinesin family (blue, gold, pink). At each side of the spindle is a pole where many microtubule ends are located. In the metaphase stage shown, sister chromatids are attached to each other (the beads from a pair are connected), but as anaphase begins a few minutes later, these bonds will break, and the chromatids will separate along the spindle axis, producing two equal genetic pools near the poles, that will be eventually inherited by the daughter cells.
Professor Dr. Arp Schnittger from the Department of Biology at University of Hamburg added: "We were surprised ourselves at how well our simulation reproduced our experimental data.
In the future, Professor Schnittger and his team want to investigate how microtubules attach to chromosomes. "This simulation will be an important tool for us and hopefully also for many colleagues in the plant field and beyond to better understand the regulation of chromosome segregation," concludes Schnittger.
Dr Nédélec said: “This comprehensive model of spindle behaviour opens the door to future discoveries about the role of cell-cycle-dependent phosphorylation in plant cells. This study underlines the importance of computational modelling in advancing our understanding of cellular biology.”
Reference
Mariana Romeiro Motta, François Nédélec, Helen Saville, Elke Woelken, Claire Jacquerie, Martine Pastuglia, Sara Christina Stolze, Eveline Van De Slijke, Lev Böttger, Katia Belcram, Hirofumi Nakagami, Geert De Jaeger, David Bouchez, Arp Schnittger (2024) The cell cycle controls spindle architecture in Arabidopsis by activating the augmin pathway, Developmental Cell.