Sainsbury Laboratory Cambridge University (SLCU) researchers, as part of a multidisciplinary international team, have uncovered a mechanism controlling subtle changes to the architecture of cell walls in plant roots that bolsters their defence against Phytophthora palmivora without negatively affecting plant growth.
Each year 30 to 40 percent of the world’s cocoa harvest is destroyed by black pod rot disease. One of the main culprits is Phytophthora palmivora, commonly known as the ‘cacao-killer’.
P. palmivora is the cousin of the more famous and better researched Phytophthora infestans – the perpetrator of the Irish Potato Famine of the 1840s. But it is P. palmivora that is now making headlines as the main culprit suspected of devastating tropical crops in many parts of the world.
P. palmivora is a generalist pathogen that infects more than 200 plant species in the tropics and subtropics, including economically important crops such as oil palm, rubber tree, citrus, pineapple, durian and papaya. This means that identifying traits in plants to enhance their resistance to P. palmivora infection could potentially benefit not only chocolate-producing cacao trees, but also many other crops.
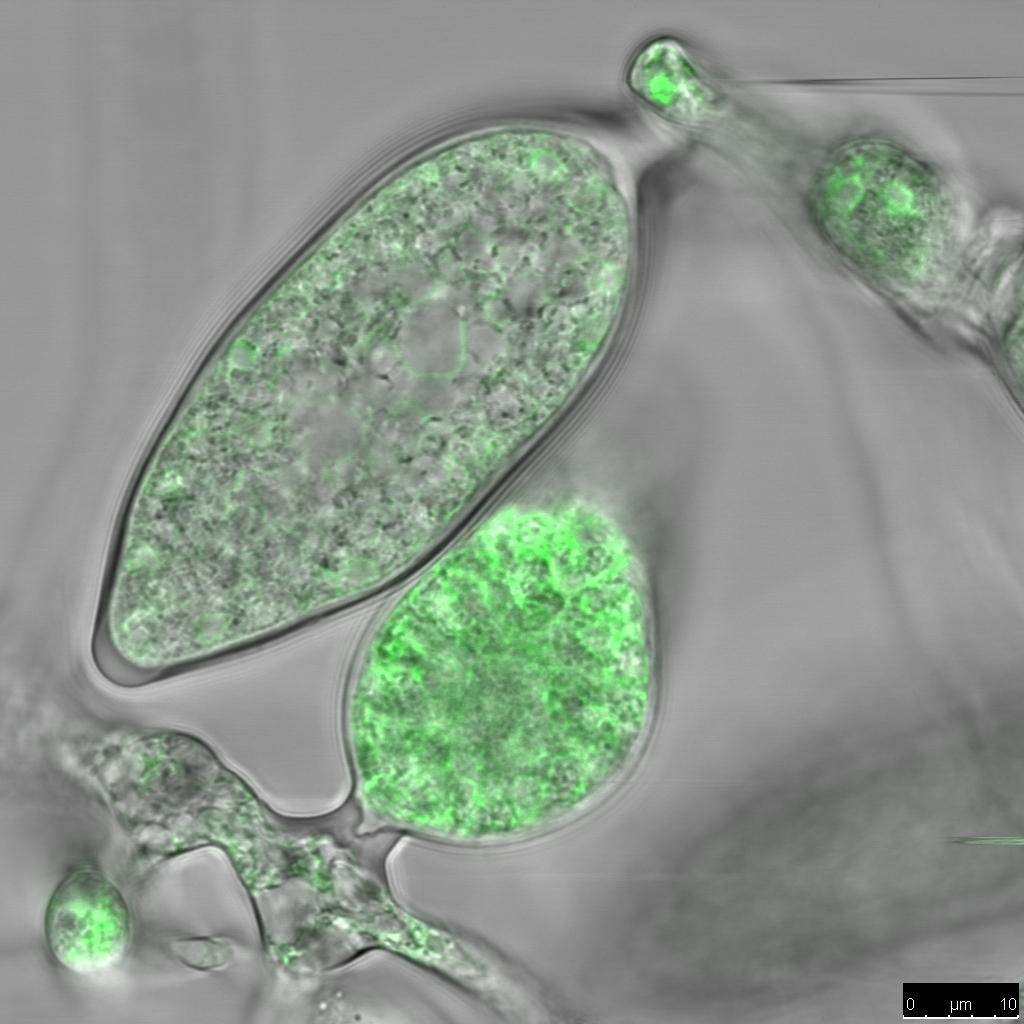
A viable strategy for resistance is the removal of plant processes that support pathogen infection, but it is important that this does not affect overall plant growth and development, and consequently crop yield.
Sainsbury Laboratory Cambridge University (SLCU) researchers, as part of a multidisciplinary international team, have uncovered a mechanism controlling subtle changes to the architecture of cell walls in plant roots that bolsters their defence against P. palmivora without negatively affecting plant growth.
Plants employ various methods to resist pathogens, but their first line of defence is their cell walls – a layer of tough cellulose and other structural support polymers surrounding each plant cell. However, the cell wall is not an impenetrable barrier. It has a complex structure that allows plant cells to grow and develop, allows the movement of water and nutrients between cells, and permits entry of beneficial organisms. The downside of this is that pathogens can also enter.
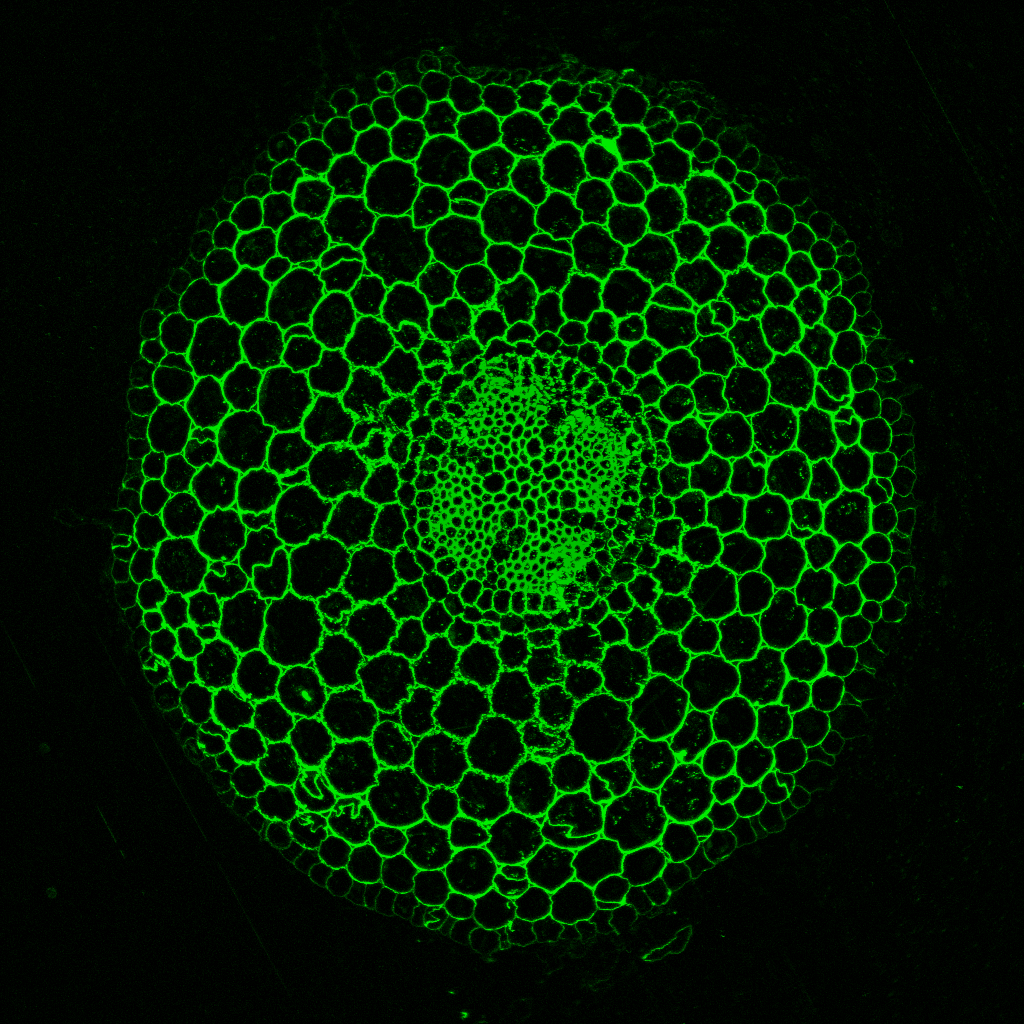
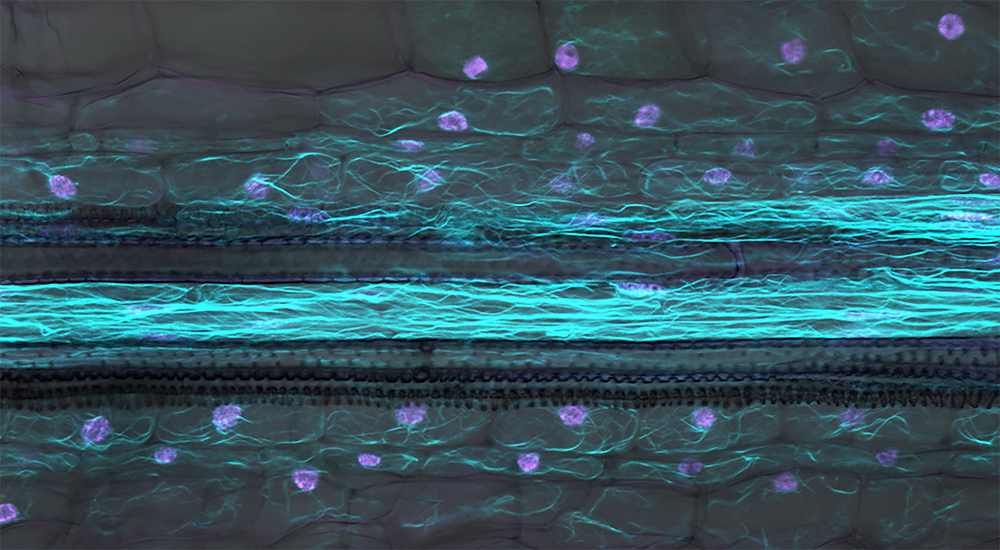
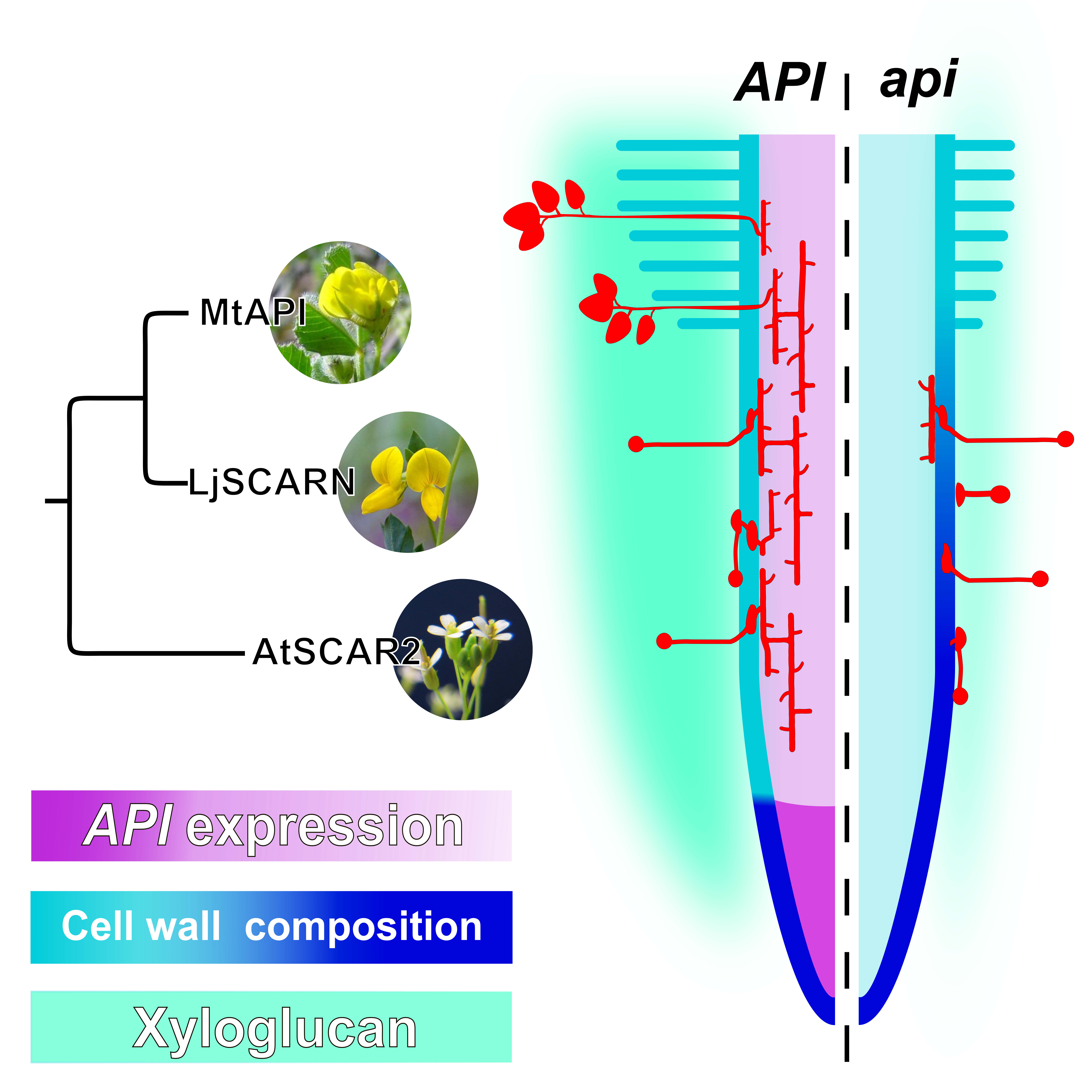
“We showed that plants which cannot produce a protein called API have subtle changes in the architecture of the walls around new cells formed at growing root tips,” said SLCU’s Dr Aleksandr Gavrin who led the research published in Current Biology today. The changes to cell wall architecture seem to impair pathogen entry to the root-tip from the invading pathogen Phytophthora palmivora, without massively altering overall plant development.
“The root tips of plants depleted of API have altered cytoskeleton dynamics, which affects trafficking and secretion of the cell wall polysaccharide xyloglucan. We identified that the absence of this single gene alters biochemical properties in the cell wall and hinders P. palmivora infection progress.”
|
What is API? API is a member of the SCAR/WAVE protein family found in both animals and plants. SCAR proteins are involved in helping cells reorganise their cytoplasm and change shape in response to growth signals. API and related proteins employ filaments that form an internal skeleton and serve as transport highways within cells. In this way, they also help microbes to infect plants – both symbionts and pathogens. API controls actin and endomembrane trafficking dynamics within cells. This research found that a mutation in the gene encoding the API protein causes delayed actin and endomembrane trafficking dynamics most prominently in cells near the root tip. This results in changes to the biochemical properties of surrounding plant cell walls that likely hinder infection by the oomycete pathogen P. palmivora. |
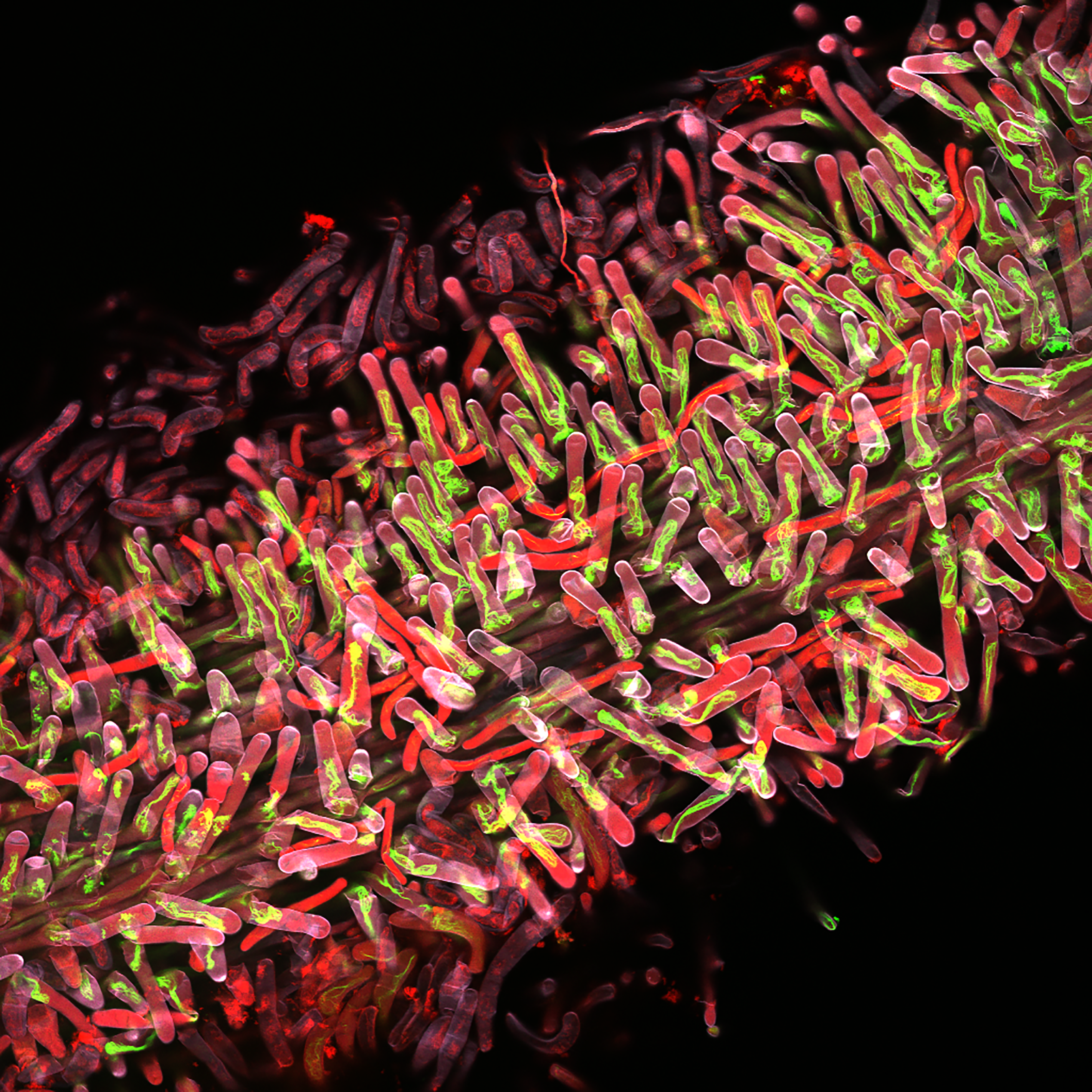
Dr Sebastian Schornack’s team originally identified the inactivation of API gene as a potential quantitative resistance trait, visible as a reduction in disease rather than an absence of it, while screening a legume species (Medicago truncatula) for mutations that impaired its ability to form symbiotic interactions with nitrogen-fixing bacteria.
“This new understanding on how quantitative traits such as cell wall architecture contribute to a plant's susceptibility to pathogens extends our repertoire of possible engineering strategies for crop fortification. The cell wall is the unique feature of all plants. It forms a barrier against invaders and therefore understanding cell wall traits could be crucial in fortifying plants against root pathogens”, Dr Schornack said.
“This work showcases how cross-disciplinary research offers new leads for crop protection strategies as it brought together the unique expertise in plant microbe interactions from Aleksandr Gavrin, Thomas Rey and others in our team, as well as plant development and cell wall knowledge from Thomas Torode, Siobhan Braybrook and others from her team.”
Journal Reference
Aleksandr Gavrin, Thomas Rey, Thomas A. Torode, Justine Toulotte, Abhishek Chatterjee, Jonathan Louis Kaplan, Edouard Evangelisti, Hiroki Takagi, Varodom Charoensawan, David Rengel, Etienne-Pascal Journet, Frédéric Debellé, Fernanda de Carvalho-Niebel, Ryohei Terauchi, Siobhan Braybrook, and Sebastian Schornack. (2020) Developmental Modulation of Root Cell Wall Architecture Confers Resistance to an Oomycete Pathogen. Current Biology, Vol 30, Issue 21.
DOI: 10.1016/j.cub.2020.08.011
Funding
This work was funded by The Gatsby Charitable Foundation, the European Research Council and by The Royal Society.





