
New insights into how buds communicate with each other through the dynamic auxin transport network have been published by SLCU plant scientists.
Most gardeners know that to encourage a bushy plant they need to prune off the top leading shoot. Decapitating the plant by removing the dominant apical shoot apex helps the many dormant lateral (axillary) buds down the main stem activate and grow into branches.
Scientists have recognised for almost a century that this effect is caused by a plant hormone called auxin, because reapplying auxin to the decapitated plant can prevent bud outgrowth. However, auxin’s inhibitory effect is indirect and influenced by a series of interactions it has with other proteins and hormones as it is transported through the shoot.
Auxin transport in the stem has mostly been attributed to the polar auxin transport stream (PATS), which provides high-capacity polar (or unidirectional) transport of auxin from the shoot apex towards the root. Ottoline Leyser’s research team has been investigating how auxin transport affects the plant’s bud activation decision-making process and previously discovered that the auxin transport network is influenced by other plant hormones, such as strigolactones, and that this interaction regulates branching. The PATS is not the only part of the auxin transport network and in 2016 they published in PLoS Biology their discovery of a more widespread and less-polar auxin transport system that feeds into the PATS and enables two-way communication between the PATS and its surrounding tissues, enabling axillary bud-bud communication. They termed this transport system connective auxin transport (CAT).
A further advance in understanding how CAT operates has now been published in PLoS Genetics. Lead author, Dr Martin van Rongen, took a genetic approach to understand how CAT impacts shoot branching control, in particular in relation to strigolactones, which usually act as inhibitors of bud outgrowth.
“We previously showed that the auxin exporters PIN3, PIN4 and PIN7 make an important contribution to CAT and that these exporters enable communication between buds,” Dr van Rongen said. “But the relationship between CAT and strigolactones was not yet clear and that is what we investigated in this paper. Strigolactones make it harder for plants to activate their buds and plants that are unable to sense or produce strigolactones are very branchy. We demonstrated that this effect at least partially depends on PIN3, PIN4 and PIN7 because strigolactone mutants become less branchy when these auxin exporters are not functioning. As expected, it does not completely prevent bud outgrowth, because other auxin exporters such as the closely related PIN1 protein, which plays a crucial role in the PATS, are still active. Instead, it shows that the plant uses many different auxin exporters to regulate and fine-tune the auxin transport network in the shoot to make decisions about which buds to activate.
“We also looked at ABCB19, which belongs to a different class of auxin exporters than the PINs, and it too can reduce branching in the strigolactone mutants. Strigolactones affect the auxin transport network in the shoot, but also regulate the expression of a transcription factor BRANCHED1 (BRC1), which is strongly expressed in the bud. High levels of BRC1 correlate with bud dormancy, so we wondered whether loss of the PINs or ABCB19 would impact on branching in plants that lack BRC1. Surprisingly, there was a clear difference between these two classes of auxin exporters, with the PINs having no effect on branching in brc1 mutants, but mutation in ABCB19 reducing its branching. This tells us that despite similar effects of the PINs and ABCB19 on auxin transport, they can also have very distinct roles in bud activation.
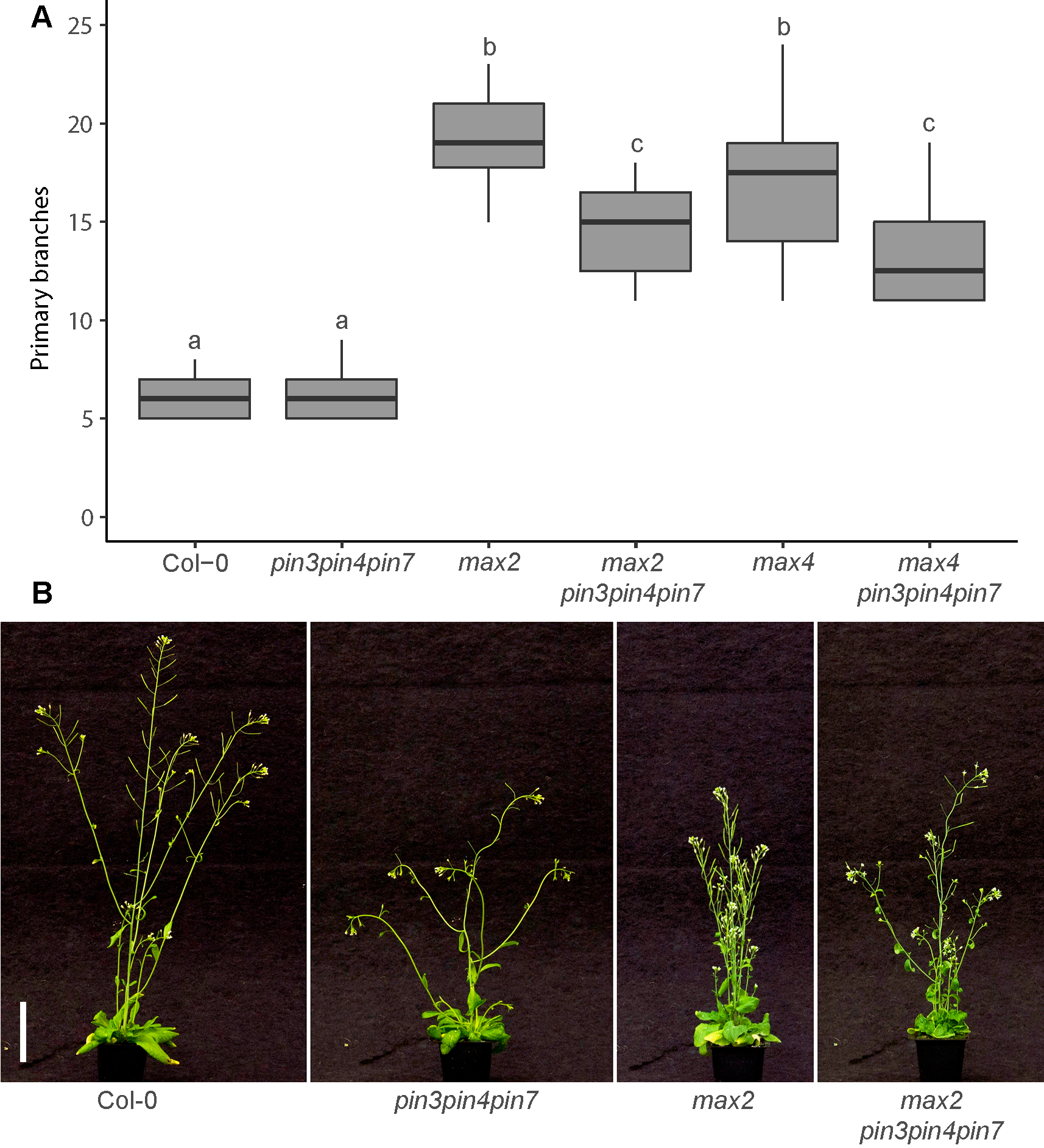
“Our current understanding of the bud activation process suggests that buds need to export their auxin out into the stem in order to activate. We think this happens through a process called auxin transport canalisation, where a broad flow of auxin gets increasingly narrower and stronger in the direction of the flow, because of the positive effect auxin movement has on its own transport. In this model of bud activation, the activity of buds depends on how easy it is for the buds to establish this auxin export, which depends on the relative auxin source strength of the bud, the sink strength of the stem and the degree of positive feedback between auxin flux and auxin transport in the direction of the flux.
“The results from this paper suggest that establishing canalised auxin transport, and thus sustained bud outgrowth, might be differentially sensitive to the action of the PINs and ABCB19. We suspect that the PINs are more important for establishing a canalised auxin flow between the bud and the stem, whereas ABCB19 might play a more localised role in auxin loading in the bud itself.”
The auxin transport network in the shoot is subject to multiple levels of feedback and the Leyser team is using the current findings to improve their further computational models of auxin transport dynamics to help address these questions.
Martin van Rongen, Tom Bennett, Fabrizio Ticchiarelli, Ottoline Leyser. (2019) Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1. PLoS Genetics https://doi.org/10.1371/journal.pgen.1008023





