Plant scientists at SLCU and the University of Bordeaux have discovered a gene that they hope can be used to widen a nutrient trafficking bottleneck and potentially increase crop yields.
Plant scientists around the world are working on a number of different strategies to sustainably increase crop yields. Increasing the efficiency of how plants transport sugars, proteins and other organic nutrients between different parts of the plant is one of the approaches that could contribute to this next Green Revolution.
Having an understanding of factors that affect local and long distance transport within a plant could enable plant biotechnologists to breed more productive crops in the future. Ultimately, it might be possible to direct transport of organic nutrients to specific parts of the plant that are harvested (seeds, fruits and storage tubers).
Professor Yrjö Helariutta’s research team at the Sainsbury Laboratory Cambridge University (SLCU) and Dr Emmanuelle Bayer’s team at the University of Bordeaux/CNRS have brought this goal a step closer by discovering Phloem Unloading Modulator (PLM), a novel gene that affects nutrient trafficking by altering the channels connecting neighbouring plant cells called plasmodesmata. These nanoscale membrane-lined channels traverse the cell wall barrier to link plant cells together and enable the transfer of essential substances (see below box).
|
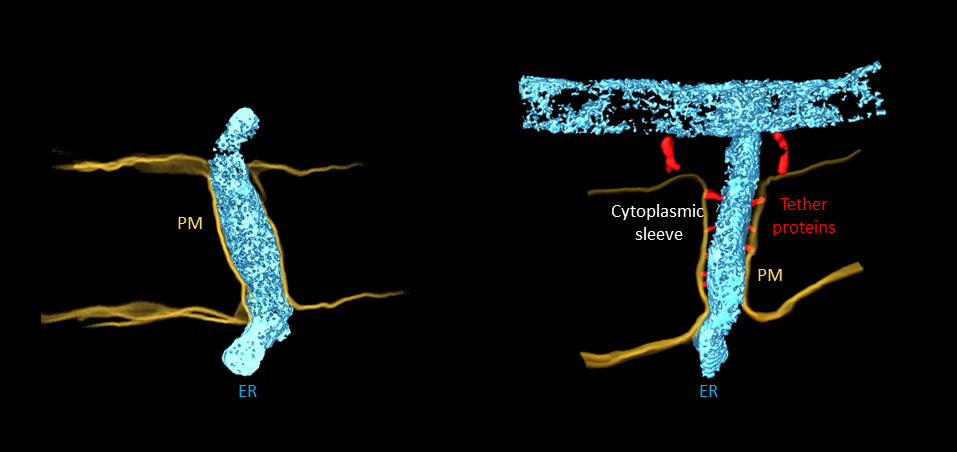
Plasmodesmata: Cell-to-cell transport channels Plasmodesmata are nanoscale membrane-lined channels that traverse the cell wall barrier to link plant cells together and through which essential substances can travel. Cell-to-cell trafficking of organic nutrients and signals via the plasmodesmata play an important role in plant growth and development, as well as disease and stress resistance. Structure Primary plasmodesmata formed in new cells are narrow single-channel structures, while secondary plasmodesmata formed in older tissues tend to be wider branched structures. However, plasmodesmata are not simple holes – they are dynamic structures that control what molecules can or cannot pass between cells based on size-exclusion principles. They are lined by a plasma membrane (PM) and contain a tubular strand of endoplasmic reticulum (ER) membrane. The gap between these two membranes is called the cytoplasmic sleeve. Previous models postulated that there was a direct link between the spacing between ER and PM and permeability, with larger gaps being more conductive. |
The study, published today in Nature Plants, shows that Arabidopsis thaliana mutant plants missing the PLM gene were found to release more substances from the phloem (a specialised tissue for long distance transport) at the tips of their roots. Using a fluorescent protein as a proxy for macromolecules, the scientists could see that the PLM gene was having a clear controlling effect on the amount of phloem unloading. To find out how the gene was doing this, they looked at what was happening at different cell interfaces in the roots of seedling plants.
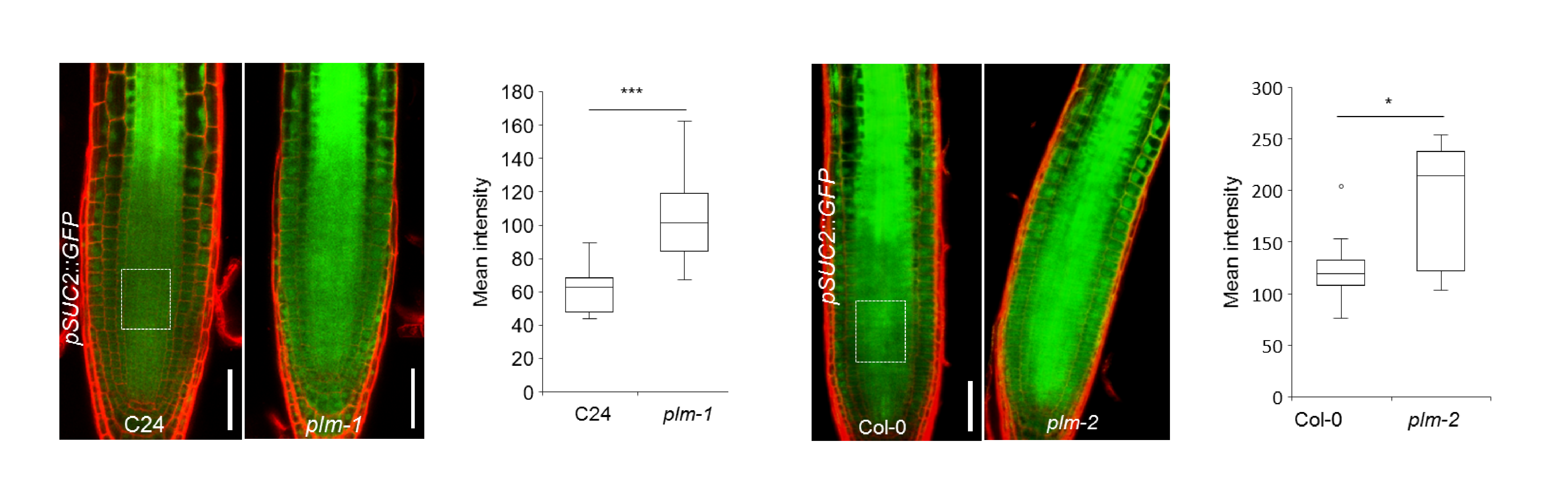 There was increased nutrient unloading in the plm mutant.
There was increased nutrient unloading in the plm mutant.
As a result of the increased unloading, the roots in the mutant plants grew faster and longer.

The plm mutant had longer roots.
Lead author, Dr Dawei Yan, from Cambridge’s Sainsbury Laboratory, explains: “We found that mutating PLM relieves a trafficking bottleneck, that was previously reducing the outward movement of nutrients from the vascular system to the rapidly growing tissues in the roots. PLM is specifically acting at the interface between the phloem pole pericycle (PPP) and endodermal cells, an interface important for the radial movement of substances after unloading. Removing PLM gene activity could enable plants to more rapidly and efficiently transport nutrients to where they are needed.”
Further molecular and genetic investigations also revealed that PLM is involved in the biosynthesis of sphingolipids, which are a class of lipids associated with plant development and response to environment. While Dr Bayer’s team had previously shown that the membranes of plasmodesmata are enriched with sphingolipids, this is the first study to link sphingolipids to plasmodesmata function.
The next step was to determine how PLM was affecting cell-to-cell conductivity. The team looked at whether PLM was influencing plasmodesmata density – it wasn’t. The team also confirmed that PLM was not modifying callose accumulation, which was the only well-known regulator of plasmodesmata permeability.
Second author and PhD student in the Helariutta group, Andrea Paterlini, went to France to work with Dr Bayer to take a closer look at whether PLM was instead affecting the plasmodesmata structure. They used electron tomography to create a 3D map of the channels at the nanometre scale.
Paterlini says: “This allowed us to detect fine alterations to the plasmodesmal architecture. We found equal proportions of simple and branched plasmodesmata in both the plm mutant and wild type plants. However, plants without PLM only had Type I plasmodesmata (see notes), rather than the two types normally found.
“Previous models have assumed that the size of the cytoplasmic sleeve (the spacing between endoplasmic reticulum and plasma membranes in plasmodesmata) would positively correlate with transport capacity. The results of our paper challenge this and show that Type I plasmodesmata, those with a very narrow cytoplasmic sleeve, are actually more conductive than Type II plasmodesmata, which have an open cytoplasmic sleeve.”
Andrea Paterlini used electron tomography to take a closer look at whether PLM was affecting the plasmodesmata structure.
|
Types A new classification system to describe plasmodesmata based on the distance between the ER and PM was proposed in Nicolas et al 2017. Type I plasmodesmata present such closely apposed membranes that no cytoplasmic space between the two is visible (narrow-sleeved). Conversely, Type II plasmodesmata have a clear ER-PM gap (open-sleeved). Despite the lack of a sleeve (regarded as the transport route in traditional models) Type I were shown to allow molecules to pass. This latest research not only supports this, but also shows that Type I plasmodesmata are more conductive than Type II.
Type I plasmodesmata (left) with closely apposed endoplasmic reticulum (ER) and plasma membrane (PM), and no obvious cytoplasmic sleeve. Type II plasmodesmata (right) with ER and PM spaced by tether proteins and visible cytoplasmic sleeve. Type II plasmodesmata are absent in plm mutants. |
Professor Helariutta says: “The correlation between the gene function (lipid biosynthesis) and the alteration in plasmodesmata ultrastructure is likely to be the result of a defect in the organisation of the tethers that separate the plasma membrane from the endoplasmic reticulum within plasmodesmata: This results in the majority of the plasmodesmata lacking visible cytoplasmic sleeves in the plants with a mutated PLM gene.
“There are still many new questions to be answered for future research, such as, how and why plasmodesmata that lack cytoplasmic sleeves have higher rates of trafficking and how the metabolism of sphingolipids is mechanistically related to the function of PLM.
“However, this research has advanced our understanding of the factors regulating plant nutrient transport. There is an urgent need to develop crops with increased nutrient efficiency, both to reduce the use of fertilisers and to increase the yield of crops. We may eventually be able to use this information about nutrient transport to more efficiently partition nutrients between various organs and direct nutrients from stems and leaves to fruit and storage organs.
Funding acknowledgements
This research was supported by the Finnish Centre of Excellence in Molecular Biology of Primary Producers; Gatsby Charitable Foundation; National Science Foundation Biotechnology and Biological Sciences Research Council; the European Research Council; Formation à la Recherche dans l’Industrie et l’Agriculture; The Region Aquitaine; FranceBioImaging Infrastructure and the DOE Joint BioEnergy Institute.
Reference
Dawei Yan, Shri Ram Yadav, Andrea Paterlini, William J. Nicolas, Jules D. Petit, Lysiane Brocard, Ilya Belevich, Magali S. Grison, Anne Vaten, Leila Karami, Sedeer el-Showk, Jung-Youn Lee, Gosia M. Murawska, Jenny Mortimer, Michael Knoblauch, Eija Jokitalo, Jonathan E. Markham, Emmanuelle M. Bayer, Ykä Helariutta (2019) Sphingolipid biosynthesis modulates plasmodesmal ultrastructure and phloem unloading. Nature Plants 5, 604–615