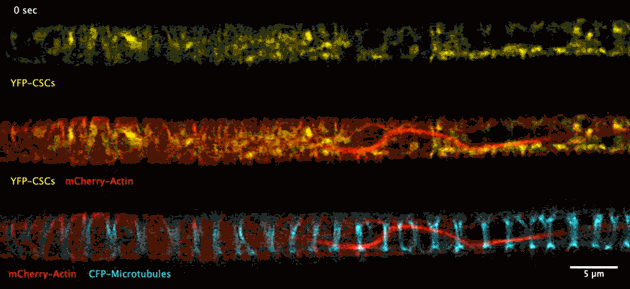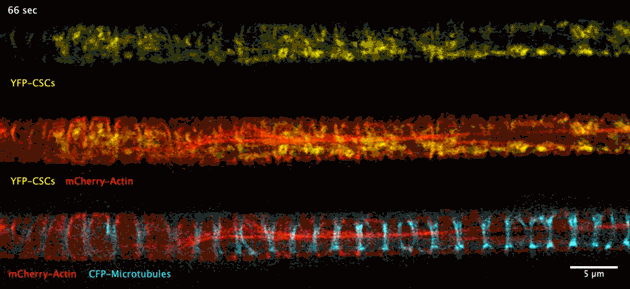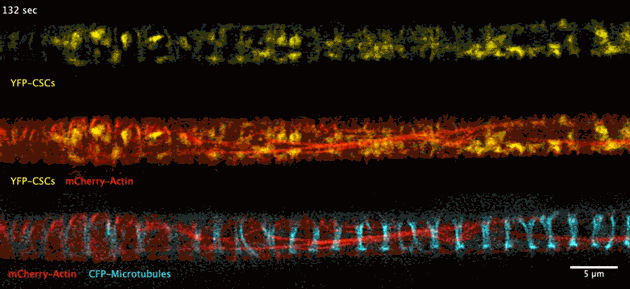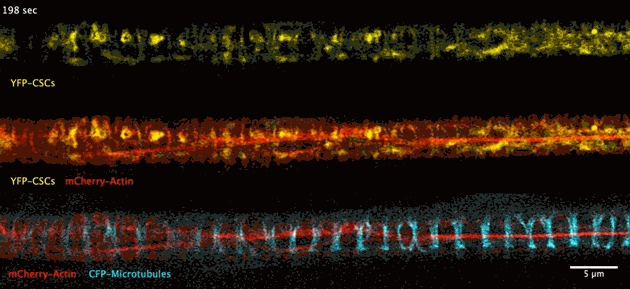
The biosynthesis of the principle component of native wood in real time has been observed for the first time thanks to advances in deep tissue super-resolution microscopy – revealing the wood-forming cells to be highly efficient material makers.
Research up until now has relied on artificially induced wood-making cells that manufacture wood in 3-4 days whereas it is believed some native wood-making cells complete this in minutes.
Wood is manufactured by specialised secondary wall-forming cells located deep within plant tissues. The cells manufacturing wood, such as xylem cells, deposit cellulose fibres directly onto the cell surface to create secondary cell walls. They do this via small cellular factories, known as cellulose synthases, that spin out the cellulose as they move along the cell surface. The vast majority of woody cellulose is made by a dedicated cellulose synthase factory. Other wood polymers, such as xylan, are incorporated in to the cellulose fibres and then the whole secondary wall is impregnated with lignin. This secondary cell wall material provides plants with mechanical support and a means to transport water long distances under high negative pressure.
It is the remarkable strength and durability of wood as a raw material that has made it a valuable resource for humans since prehistoric times. Wood is still an economically important material and increasingly being used in sustainable industry and engineering applications. As such, optimising the mechanical properties of this natural material to meet modern building and engineering standards is a major focus for manufacturers and requires a better understanding of the basic structure of wood and how plant cells microfabricate this material.
As the Imaging Core Facility Manager at the Sainsbury Laboratory Cambridge University (SLCU), Dr Raymond Wightman works with scientists to develop new microscopy tools to advance our knowledge of the inner workings of plants. One area that has fascinated him for a long time, and that he has been investigating himself, is the biosynthesis of wood.
“Until recently, we could only observe what happens at the surface of plants. We did not have the tools to see deep inside the plant to observe wood being synthesised and deposited,” Wightman said.
To solve this problem, scientists converted epidermal plant cells into artificial wood-making cells, which has revealed some important mechanisms in the synthesis of wood. It took 3-4 days for these converted cells growing on the plant surface to manufacture all the cellulose fibrils that make up a secondary cell wall. However, this artificially induced system had different cell sizes and shapes and there has long been the question of whether they make the wall in precisely the same way as native wood-making cells located deeper inside the plant.
“No one knew if cellulose synthases were working in the same way in genuine wood-making cells inside stems and roots,” Wightman added. “We needed to look at wood formation in native wood-making cells to help us to understand how plants make this amazing material. To do this we had to be able to see past the many layers of other cells to view the subcellular processes happening at the surface of xylem cells – and this is not easy to do.”
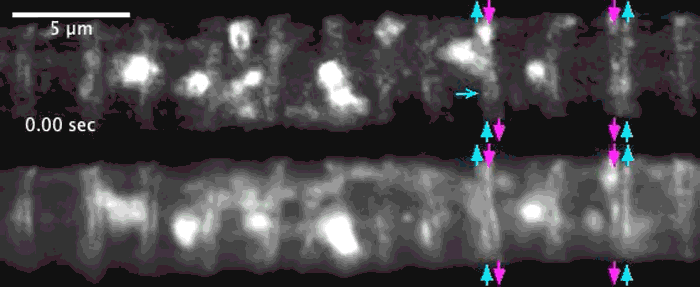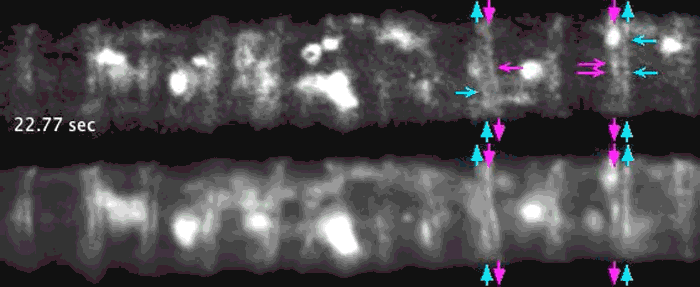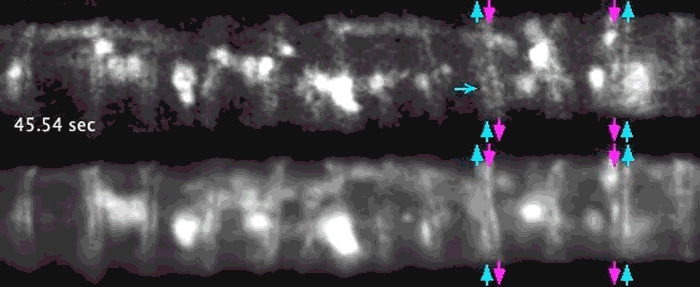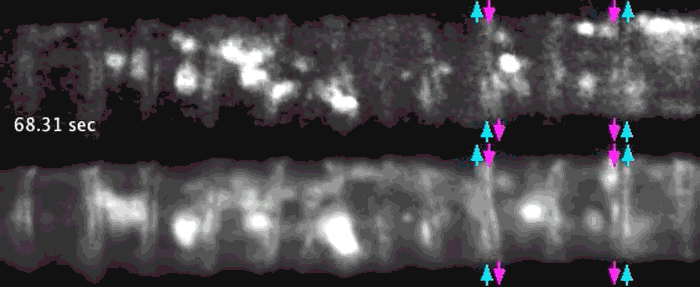
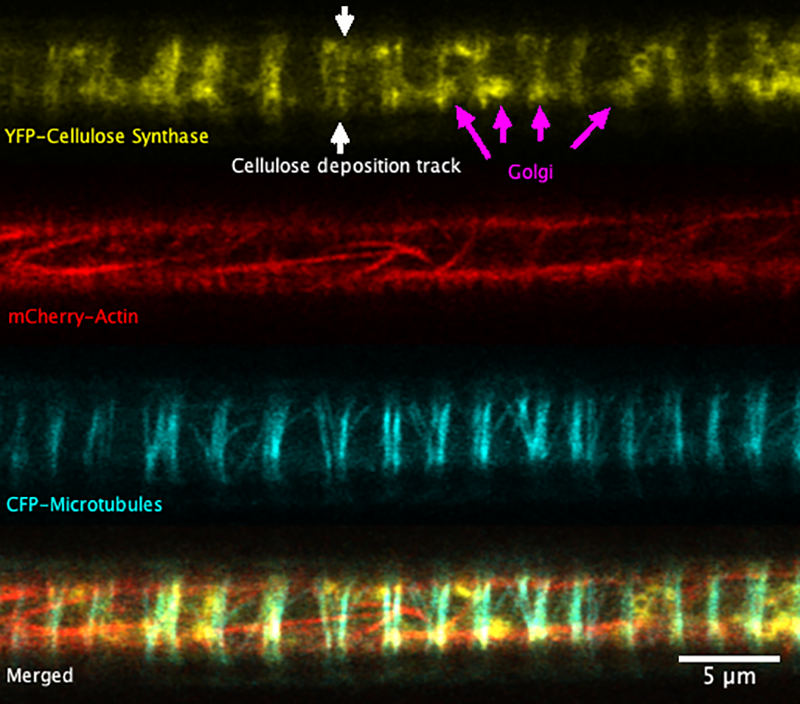
Super-resolution images deep inside the living root showing a portion of a narrow xylem vessel and fluorescently-tagged cellulose synthase complex (CSC) compartments, fluorescent actin and fluorescent microtubules that all work together to make cellulose for wood formation.
Optical microscopy using airyscanning is a technique that allows scientists to get a view deep within living tissues in real-time and obtain high resolution information close to the nanoscale. Wightman tested and honed this technique, which allowed him to track the movement of the cellulose synthase particles as the secondary cell wall was being built.
“I have been trying to solve this problem for 20 years and it is only recently that we have had the tools that allow us to see the dynamics of the cellulose synthase complex at the cell surface of xylem cells. The key tool is the airyscanning technology on an advanced laser-scanning confocal microscope that was installed at the Sainsbury Laboratory in 2018. Confocal microscopes use a pinhole to improve the resolution of light microscopy, however, the airyscan divides this pinhole in to 32 smaller chunks that improves resolution even further and improves the detection of the feint fluorescence signals emanating from the centre of the plant.”
A developing root protoxylem vessel labelled with CSCs (yellow, YFP-CESA7). The lower image is an average of the whole movie, with the cellulose synthase complex (CSC) clearly visible as vertical tracks.
Wightman found that the cellulose synthase dynamics agreed with the earlier findings from converted epidermal cells that synthesise cellulose into secondary cell walls, but with important differences. Now able to look at wood formation in much more detail he explained what he observed: “The young xylem vessels are long, thin tubes and everything inside the cell appears to be hurtling around the cell along the thick actin bundles like a fast spin in a washing machine” he said. “This needs careful co-ordination to deliver the synthases along with other wood material to all the sites on the cell surface, marked by bundles of microtubules, and to complete the process on a timescale of minutes. Working out how these cells coordinate this feat is my next challenge.”
Reference
Raymond Wightman (2023) Observing cellulose synthases at emerging secondary thickenings in developing xylem vessels of the plant root using airyscan confocal microscopy. The Cell Surface. Vol 9. https://doi.org/10.1016/j.tcsw.2023.100103