Inside every living cell, there is a network of protein filaments providing an interior scaffold controlling the cell’s shape called the cytoskeleton. Research from the Sainsbury Laboratory Cambridge University (SLCU) suggests that this relationship might actually be two-way, with cell geometry itself having the capacity to influence the organisation of the cytoskeleton in living plant cells.
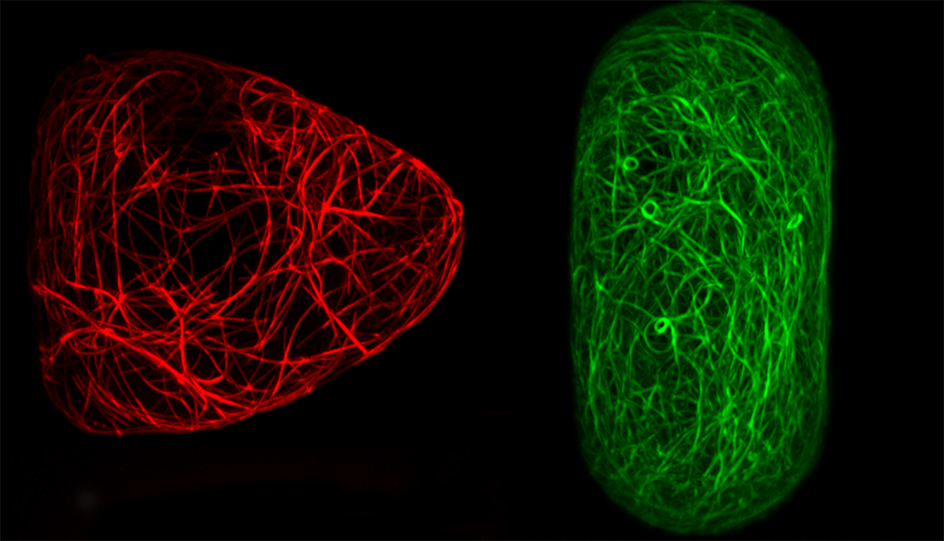
Living cells come in all sorts of shapes. The shape of a cell reflects its role and assists the cell to carry out specific biological functions. For example, nerve cells have long thin extensions that facilitate messaging between other nerve cells; some bacteria have whip-like tails protruding from their cell to drive locomotion; the hairs or spikes on single-celled pollen grains help them stick to pollinators like bees; pairs of bean-shaped guard cells open and close pores on plant leaves to control gas exchange and minimise water loss, and the intricate and beautiful shapes of diatoms (single-cell algae) enhance nutrient uptake and help them float across the seas.
This enormous variety of cell shapes are all controlled by a dynamic network of protein filaments within each cell called the cytoskeleton. The cytoskeleton behaves like the cell’s ‘skeleton’ to form a framework, but this framework is not static and is continually moving and changing within a cell.
“To understand how the cytoskeleton gives cells their shape, and consequently influences the architecture of whole tissues, we need to identify how the cytoskeleton filaments move and interact with each other to form this network within a cell,” said Dr Pauline Durand-Smet from the SLCU, who led the research published in . “As cells multiply and expand to form tissues they exert mechanical stress on their neighbours, influencing cell shape and the eventual architecture of the tissue or organ. Mechanical forces and cell geometry have been shown to influence the cytoskeleton in animal cells, but this has been less characterised in plants and so I wanted to investigate whether cell shape also influences cytoskeleton organisation in plants. We hoped to see whether the alignment of the cytoskeleton filaments was affected by changing the geometry or shape of a plant cell and from this gain a deeper insight into how the two major cytoskeleton filaments, actin and microtubules, interact with each other.”
To conduct the research Dr Durand-Smet designed and then used micro-lithography to manufacture a set of tiny silicone moulds in circle, square, rectangle and triangle geometries. Each mould was used to make a sheet of agar with precisely sized micro-wells that could each hold a single plant protoplast (plant cell without a cell wall).
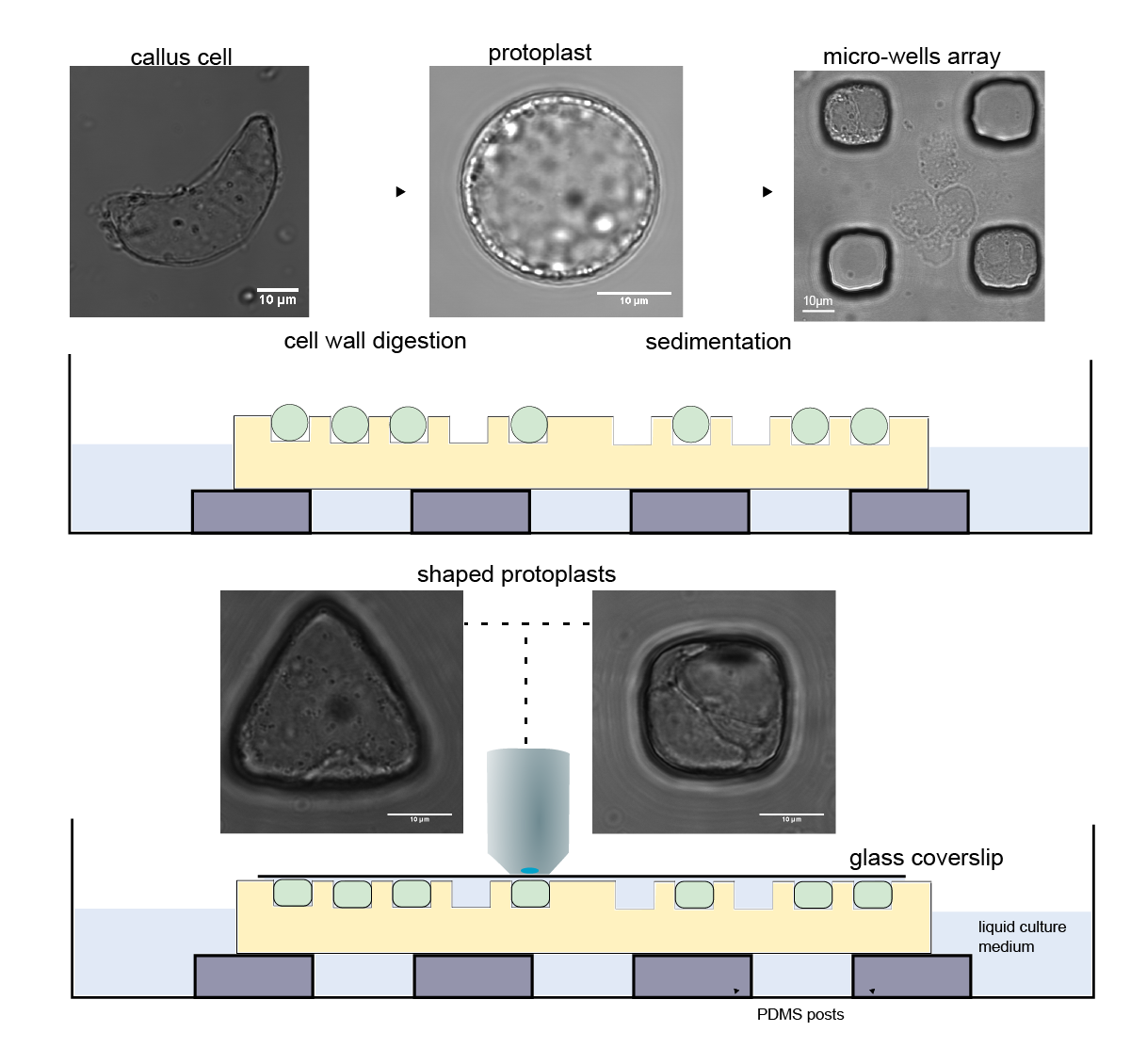
The process involved carefully balancing the osmotic pressure within the protoplasts to prevent them from bursting while pouring the protoplast mix into the mould and then selecting the single protoplasts that had slotted precisely into a well to study further. Adding to this challenge was the need to undertake the cytoskeleton observations quickly within a few hours before the plant cell started to regenerate its cell wall.
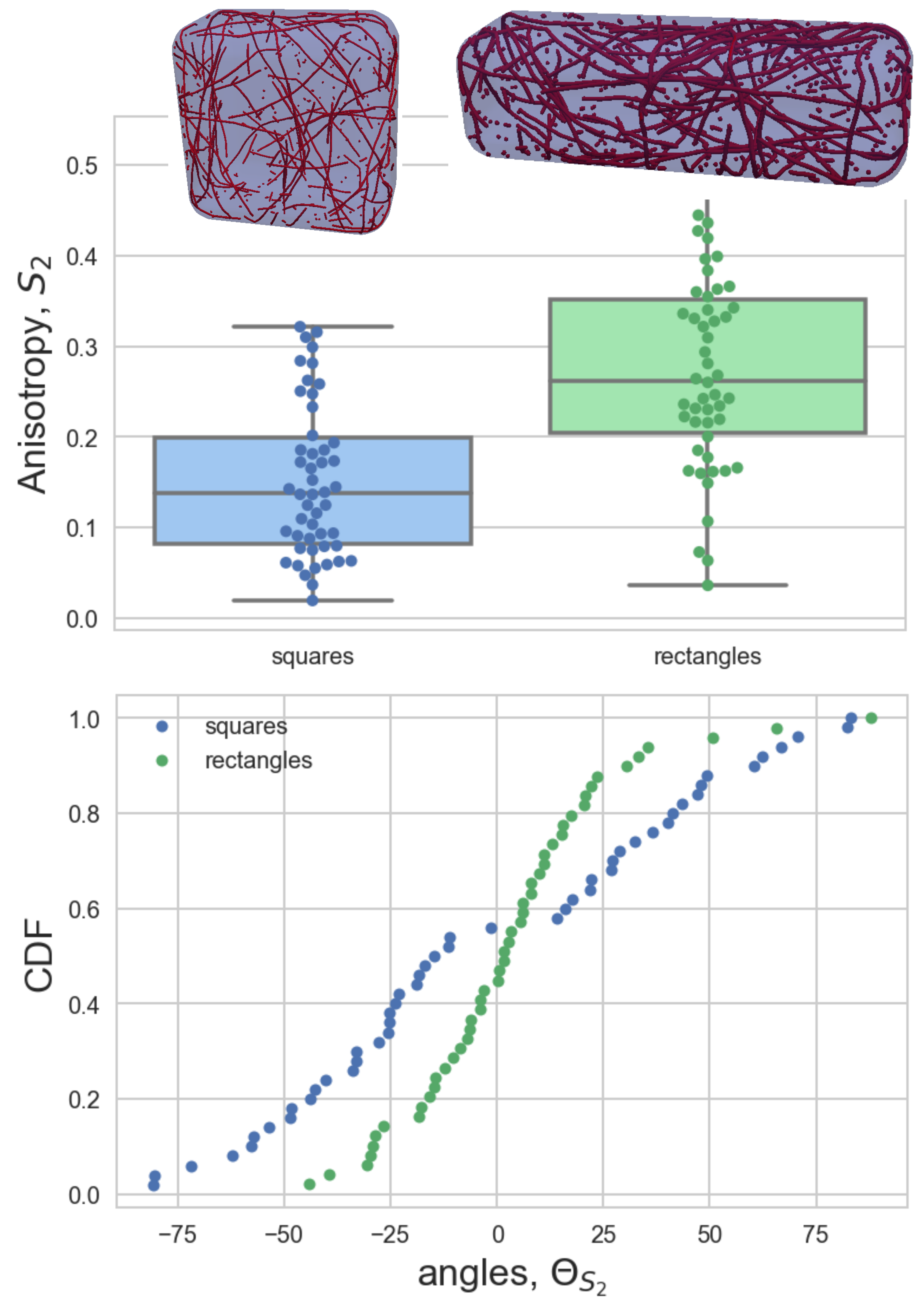
Fluorescent marker proteins were used to make the actin and microtubules visible, which allowed precise quantification of the cytoskeletal organisation with high-resolution microscopy. This research is one of the very few studies to look at actin and microtubules together in the same experimental set-up. Microtubules are more rigid than actin and so the researchers expected to see different organisation between the two networks with microtubules aligning in longer cell geometries like the rectangle shape.
“We quantified the orientation of the filament network by measuring the average angle of the filaments and how well the filaments are aligned between one another,” said Durand-Smet. “Our study showed that actin filaments and microtubules both interacted in response to different cell geometries. To our surprise, both actin and microtubules aligning along the long axis of cells constrained in rectangle shapes.”
The researchers think that this organisation is the signature of self-assembling networks. “By studying actin and microtubules in cells with the same system we showed that while the alignment between actin filaments depends on the organisation of the microtubules the converse is not the case. By genetically modifying the interaction rules between the microtubules, we observed that the alignment of microtubule filaments was reduced.”
Fellow SLCU researcher, Dr Tamsin Spelman, adapted a 3D model to explore whether the experimental results could be explained by the impact of cell geometry. “We extended the model of an evolving 3D microtubule network to predict the role of microtubule severing on alignment within different shapes,” Dr Spelman said. “The model showed that the observed organisation of microtubules in the cuboid shape is dependent on filament severing. The cell cytoskeleton may be a biological system but its behaviour still has to obey the laws of physics.”
Dr Durand-Smet said this work is a first step towards assessing quantitatively how cell geometry contributes to the control of the cytoskeletal organisation in living plant cells. “Our experiments and modelling suggest that microtubules have a dominant role in influencing the cytoskeletal organisation in response to cell shape. We hope that this approach can be further developed to study how geometry impacts plant cell division or cell polarity.”
The researchers plan to continue their cytoskeleton investigations by tracking how the cytoskeleton re-aligns during the cell shape-transition and measuring the impact of a quantifiable force applied to a cell.
Reference
Pauline Durand-Smet, Tamsin A. Spelman, Elliot M. Meyerowitz and Henrik Jönsson. (July 2020) Cytoskeletal organization in isolated plant cells under geometry control.
Funding
This work was supported by a Marie Sklodowska-Curie fellowship, Gatsby Charitable Foundation, HFSPO and the Howard Hughes Medical Institute.





