New findings explain how the uncurling of the apical hook in illuminated Arabidopsis thaliana seedlings relies on synchronising hormone, cytoskeletal, and cell wall dynamics to drive this growth process.
Using a plant seedling apical hook as a model for organ shape change, research conducted at the Sainsbury Laboratory, University of Cambridge, shows how plants strike a balance between speeding opening and mechanical integrity for the seedling stem to transition from a curved to straight form.
Light-induced hook opening of the hypocotyl, expressing a plasma-membrane marker (LTI6b-YFP), using a laser scanning confocal microscope, with illumination on the seedling between image acquisitions stimulating apical hook opening. Images were acquired at 30 min intervals over a period of 8 h. Images by Ankit Walia.
When plants germinate from their seeds and begin growing through the soil in the dark, the top of their stem has a distinct curved shape, known as the apical hook. This hook helps protect the delicate cotyledons (baby leaves) and apical meristem (cluster of stem cells at the top of the plant shoot) that are responsible for future growth. As the seedling breaks through the soil surface and is exposed to light, the hook straightens out.
To appropriately transition from growth restriction to cell elongation on the concave side of the stem, the plant integrates the hormone auxin, pH, microtubule organisation and cell wall mechanical properties. These findings provide a model that helps us to understand how seedlings can actively navigate through soil and open their leaves to the light at just the right time. This is particularly important as seedling establishment is when the plant is at its most fragile and can be the difference between crop success or failure.
All organisms must adopt shapes appropriate to their environments, and plants have the added complication that their flexibility and movement is restricted by rigid cell walls that surround every one of their cells. This means plant cells can’t move about and need to integrate genetic and molecular signals with mechanical stresses from cell walls to take the right shapes in service of forming the right organ shape.
Published online in the journal Developmental Cell, Dr Alexander Jones’ research team, in multidisciplinary collaboration with Professor Elliot Meyerowitz and computational scientists in Professor Henrik Jönsson’s team, combined quantitative imaging, genetics and modelling to reveal the interplay between hormone gradients, tissue mechanics and cell wall remodelling required for the uncurling of the apical hook.
Dr Ankit Walia* and Dr Ross Carter* quantified cellular growth during this transition and found cells on the inside of the apical hook expanded by four times their original length. Induced cell deformation by osmotic treatments revealed that there are similar pressure-driven longitudinal tensional forces on the inner and outer side of the hook and that opening is a result of differential capacity for irreversible extension.
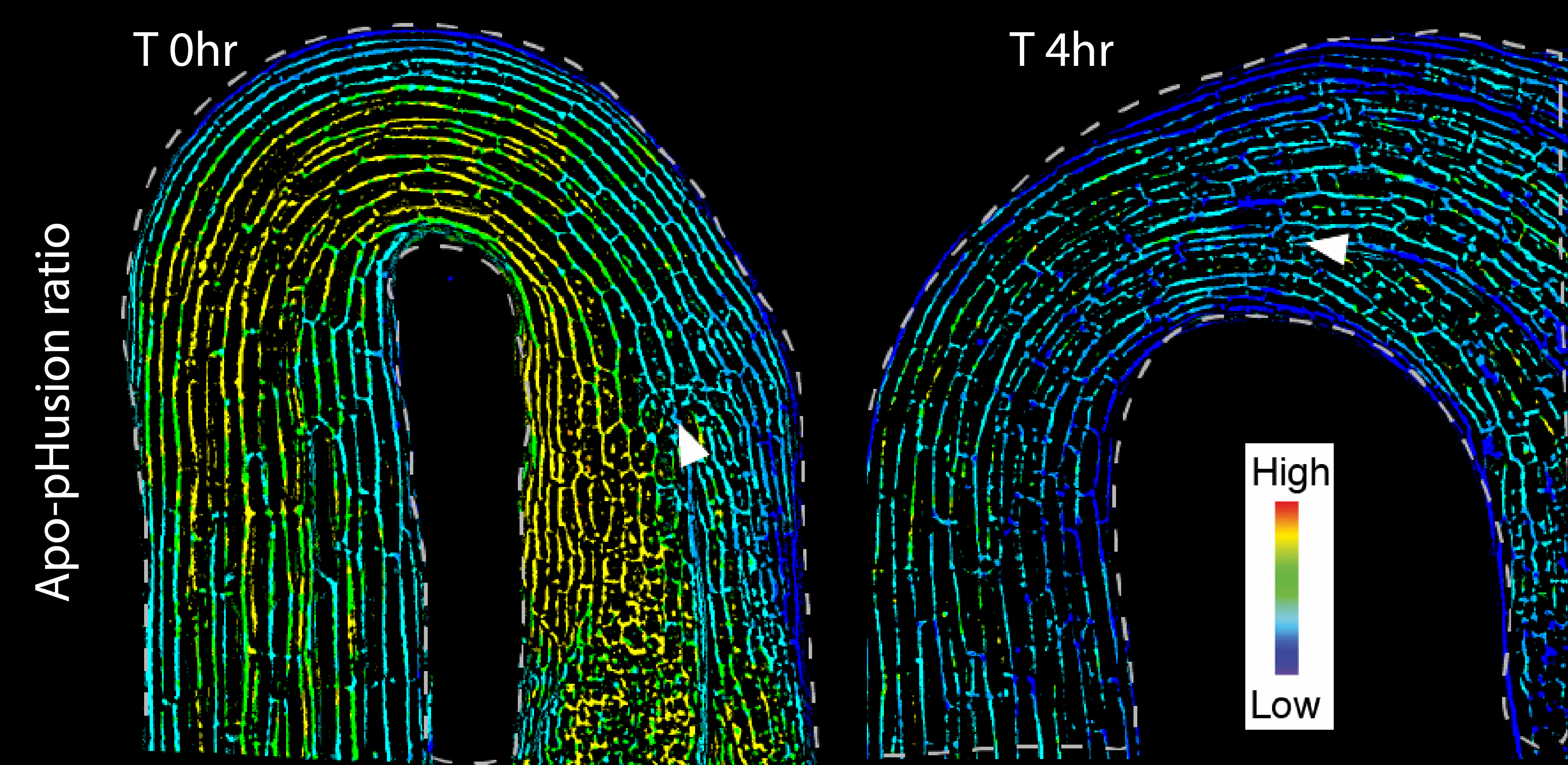
It was already known that the plant hormone auxin, a major growth regulator, accumulates on the inner side of the apical hook in the dark and that auxin signaling decreases during light exposure as the hook opens. The acid-growth theory was a long-standing hypothesis that explained how auxin promotes cell elongation by acidifying and loosening plant cell walls. But in apical hooks, high auxin works the other way – by promoting higher pH and preventing growth. For hook opening, it was not clear how auxin effects are integrated with apoplastic pH to control cell elongation.
“We found a correlation between auxin response and higher pH on the inner side of the hook in the dark,” Dr Walia said. “On exposure to light, we saw a decrease in auxin response in the inner side, which correlated with decreased pH. However, since pH depletes broadly, there may be auxin-independent post-illumination acidification effects.”
In addition, pH measurements and osmotic treatments on plants with elevated auxin levels revealed that auxin can antagonise irreversible cell expansion in all hook cells. These results indicate that auxin and high pH gate growth, but do not explain differential extension required to unhook.
Changes in apoplastic pH with growth and hook opening. Images by Ankit Walia.
But this was just one part of the story. For cells to expand in the right direction for the apical hook to open they need further information.
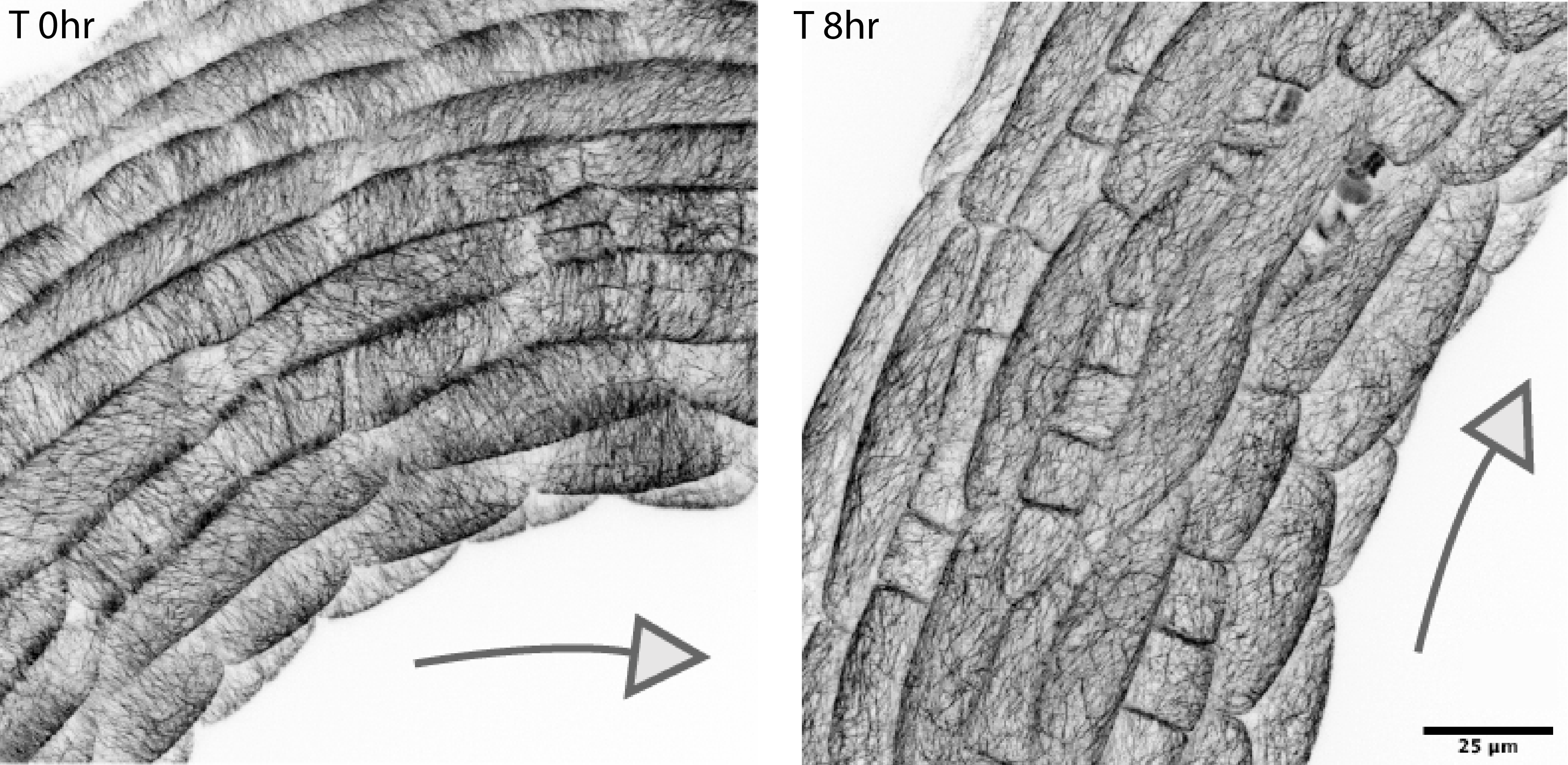
Cortical microtubules (CMTs) are part of the cytoskeleton and act as internal scaffolding within a cell that controls the orientations of cellulose microfibrils in the wall. Thus, they determine the changes in cellulose-based wall anisotropy and the direction of cell wall yielding under internal turgor pressure.
Cortical MT (CMT) orientations during apical hook opening. Images by Ankit Walia.
To gain insights into what the CMTs were doing during apical hook opening, Dr Walia tracked their orientation and found patterns that correlate with differential cellular growth happening in the apical hook. Dr Walia explained: “Circumferential CMT orientation in dark was consistent with the geometry-derived circumferential principal stress directions and their reorientation from circumferential to longitudinal post-illumination suggest an increase in longitudinal stress to which stress-responsive CMTs might be realigning on the outer face of the epidermal cells.”
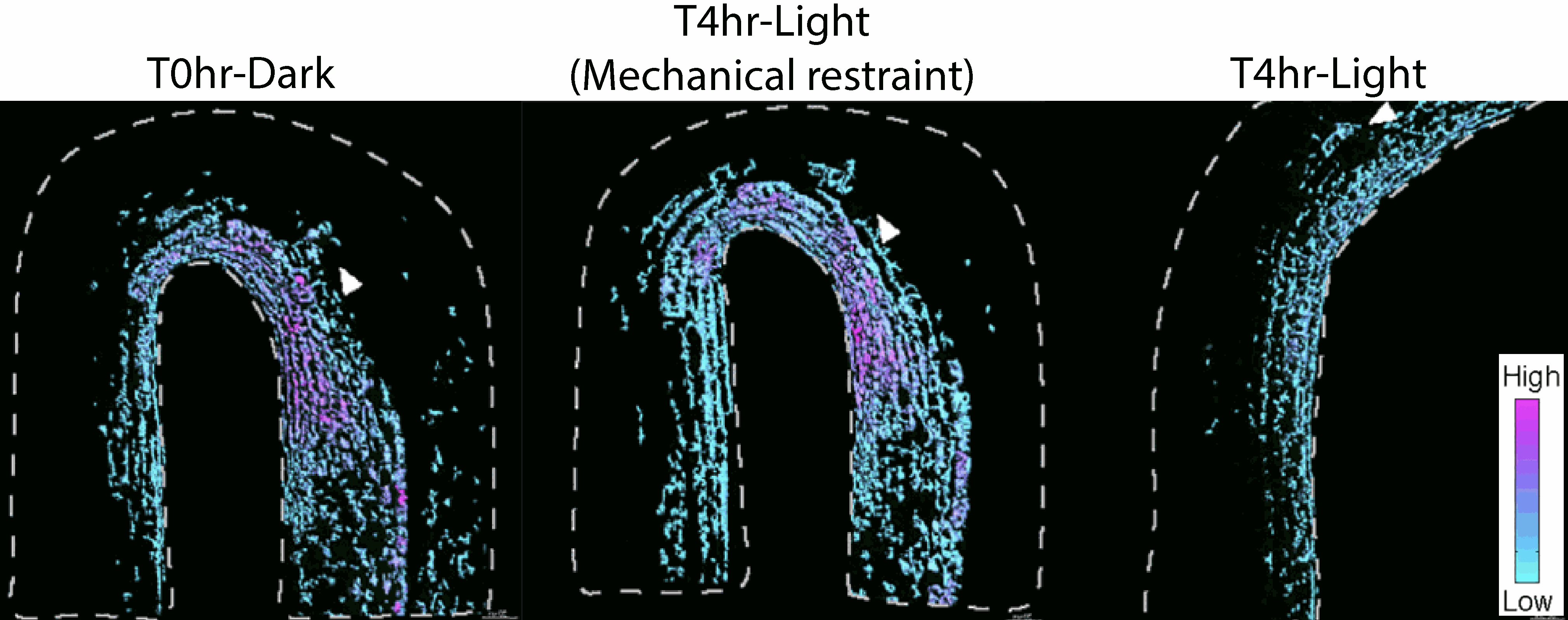
Wall mechanics feed back into cellular auxin homeostasis control. Images by Ankit Walia.
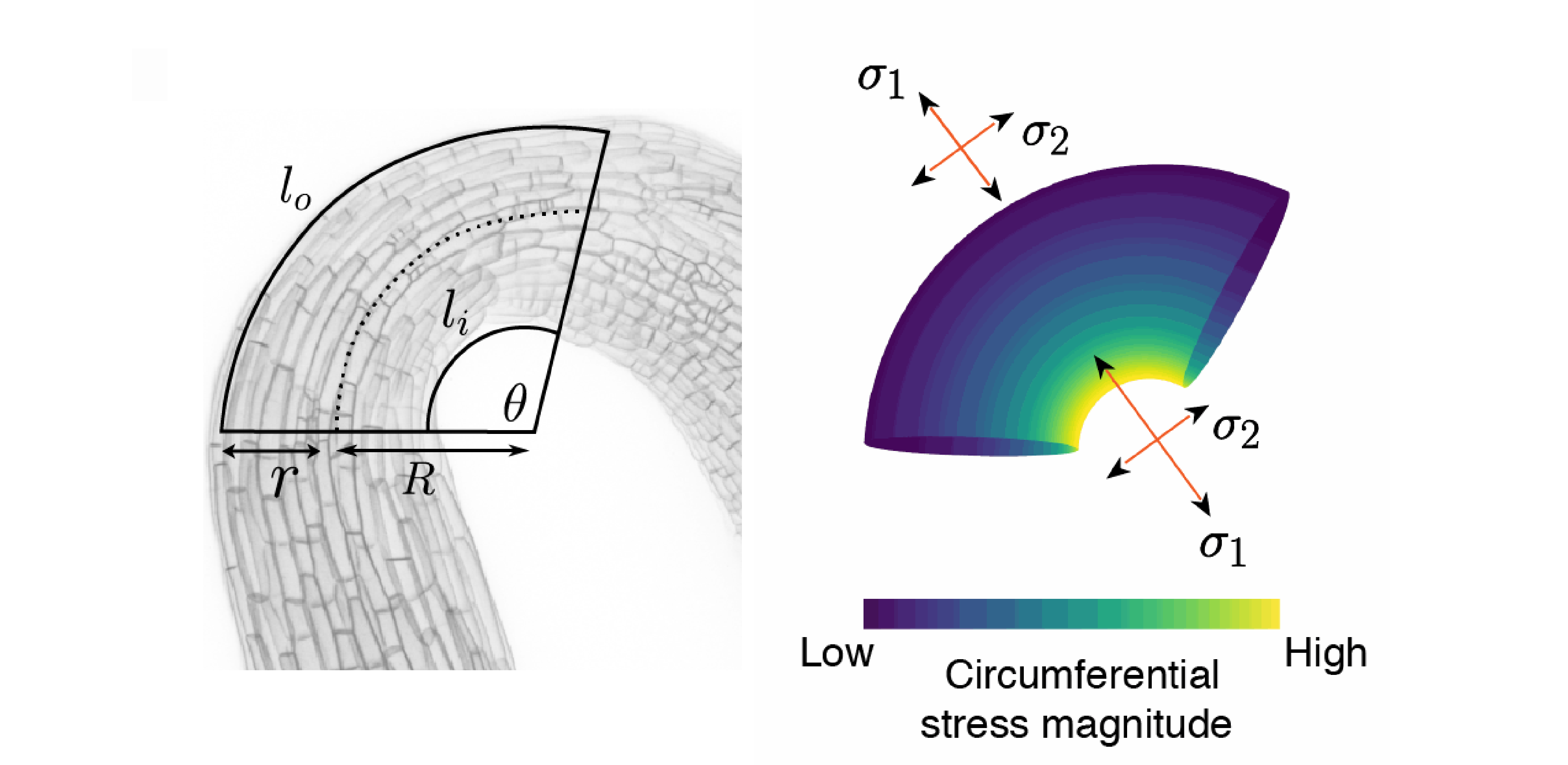
To better understand the mechanical stresses and strains acting in the apical hook, Dr Ross Carter* used this experimental data to develop a mechanical model to recreate the apical hook during opening. A key part of recreating the hypocotyl conditions was integrating previous experimental work showing that the epidermis of the hypocotyl is under tension whilst the sub-epidermal tissues are under compression. Dr Carter explained: “When you bend a hose with water flowing through it, two opposing forces act on its surface – the inner surface is compressed while the outer surface is stretched. Using the hypothesis that the underlying tissue is applying a longitudinal force on the epidermis post-illumination, we added an increasing longitudinal stress to the model. Not only did this force cause the microtubule array to switch orientation like we had observed experimentally, it also accelerated opening. Coupled with the anisotropic nature of the cell wall, we hypothesise that this behaviour has been co-opted to coordinate growth between tissue-layers and strike a balance between speedy opening and mechanical integrity.”
Toroidal pressure vessel model and circumferential and longitudinal stresses in the hook. Image by Ross Carter.
Dr Jones said the results provided a new view of the mechanochemical environment in play during differential growth in the hypocotyl: “We are using the apical hook opening as a model system to investigate mechanochemical regulation of differential growth in plants. These findings show how plant cells coordinate growth non-autonomously among tissue layers by linking mechanics, hormone gradients and cell wall remodelling required for growth. Some of the properties in our model are not easily explained with known biological factors, so there’s new mysteries to pursue.”
The work further identified mechanochemical feedback from wall mechanics to light stimulated auxin depletion, which may act to prevent hook opening under mechanical restraint. This suggests that changes in wall properties and wall mechanics are integrated with light-induced cellular auxin depletion, allowing plants to choose the most appropriate shapes for both the physical and light environment.
Profesor Jönsson summarised the significance of the findings: “This mechanochemical feedback loop is likely to have biological relevance. It is an advantage to the plant to keep its apical hook bent to protect the meristem and cotyledons as the seedling pushes up through the top layers of soil in the dark. Once it reaches light, it triggers wall acidification and auxin depletion on the inner side combined with an increase in longitudinal force in the subepidermal tissues that drives differential growth. This allows growth coordination across the tissue-layer required for differential growth in a curved organ.”
Reference
Ankit Walia*, Ross Carter*, Raymond Wightman, Elliot M. Meyerowitz, Henrik Jönsson and Alexander M. Jones (2024). Differential growth is an emergent property of mechanochemical feedback mechanisms in curved plant organs.
https://doi.org/10.1016/j.devcel.2024.09.021
*Ankit Walia and Ross Carter contributed equally to this research
Preview (Developmental Cell)
Andrew C. Willoughby and Lucia C. Strader (2024). Apical hook opening of plant seedlings: Unfolding the role of auxin and the cell wall.
https://doi.org/10.1016/j.devcel.2024.11.018