I am investigating how physical changes in cell morphology give rise to above-ground plant organs such as leaves and flowers. These organs are derived from a continuous pool of undifferentiated cells within the shoot apical meristem. I am interested in how the cells of the meristem undergo proper transformations in shape and size which is a result of changes in cell wall deposition and remodelling of existing cell wall components.
1. Cell wall formation and remodelling.
There are several poorly understood cell wall biosynthetic enzymes that appear to have a crucial role in maintaining the size and shape of the shoot apical meristem. This suggests that the cell walls in the meristem have important differences to those of the rest of the plant. We have deduced the chemical linkages within the meristem cell wall and where they occur. We are now looking at how particular wall components affect stem cell maintenance and plant growth with an emphasis of how the wall foundations are laid down during cell division. In turn wall remodelling enzymes are required to maintain the shape of the shoot apical meristem and to alter mechanical properties locally to generate new organs. We are looking at the role of a group of "cut and paste" enzymes in plant development with a focus upon the plant apex. These enzymes can cut and reattach certain structural polysaccharides within the wall as well as incorporating newly formed wall material into the existing matrix. The data from this project will be used to update 3D computer models of the shoot apex.
2. Developing new microscopy tools for the plant sciences.
As our knowledge of the inner workings of plants increase, so does our demand for new tools to take our research to the next step. I work with other plant scientists at SLCU and staff from the Cambridge University Botanical Gardens to develop improved tools for probing all aspects of plant biology. This includes:
(i) cryoSEM techniques for looking at tissue organisation and the ultrastructure of cell walls, membranes and organelles.
(ii) Correlative FLIM-confocal-Raman microscopy for observing protein-protein interactions, 3D cellular organisation and distribution of molecular species ("chemical imaging").
(iii) Developing new tools for cell culture and tissue engineering.
3. Morphology of alpine plants
In collaboration with Cambridge University Botanical Gardens, we are using new microscopy techniques to look at how alpine plants have adapted to their high altitude environments. Some Saxifraga species deposit a white or silvery crust on their leaf surfaces and we have determined the ultrastructure of the leaf glands that produce the crust and determined what the crust is made of. This led to the discovery of a rare mineral polymorph, called vaterite, that forms the crust of a subsection of species of the Saxifraga. Vaterite is not normally stable on earth and, while it is a desirable product for industry, it is difficult to produce. This new source of naturally occuring vaterite could have applications for drug delivery and new biomaterials.
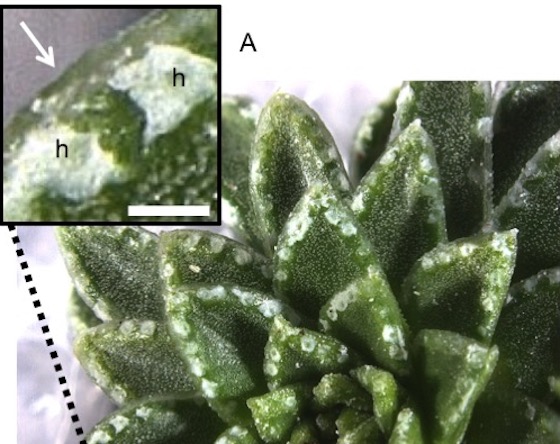
Above image: Leaves of Saxifraga scardica have patches of white crust that contains the rare mineral vaterite. Scale bar = 1 mm.
Current Collaborations
- Jönsson lab, Sainsbury Laboratory
- Dupree lab, Department of Biochemistry, Cambridge
- Peaucelle lab, INRA, France.
- CUBG
Key Publications
Wightman, R., Wallis, S. and Aston, P. Leaf margin organisation and the existence of vaterite-producing hydathodes in the alpine plant Saxifraga scardica. Flora (2018) 241: 27-34. <link to “Editors’ choice” http://science.sciencemag.org/content/360/6384/twil >
Yang, W., Wightman, R. and Meyerowitz, E.M. Cell Cycle Control by Nuclear Sequestration of CDC20 and CDH1 mRNA in Plant Stem Cells. Molecular Cell (2017) 68: 1108-1119. <Link to https://sainsbry.migrate.drupal.uis.cam.ac.uk/news/cell-division >
Wightman, R., Wallis, S. and Aston, P. Hydathode pit development in the alpine plant Saxifraga cochlearis. Flora (2017) 233: 99-108. <Link to http://www.botanic.cam.ac.uk/Botanic/NewsItem.aspx?ix=298 >
Luo, C.J. and Wightman, R. From mammalian tissue engineering to 3D plant cell culture. The Biochemist (September 2016)
Kumar, M., Wightman, R., Atanassov, I.,Gupta, A., Hurst, C., Hemsley, P. and Turner, S.R. S-Acylation of the cellulose synthase complex is essential for its plasma membrane localization. Science (2016) 353:166-169
Yang, W., Schuster, C., Beahan, C.T., Charoensawan, V., Peaucelle, A., Bacic, A.,Doblin, M.S., Wightman, R*. and Meyerowitz, E.M. Regulation of Meristem Morphogenesis by Cell Wall Synthases in Arabidopsis. Current Biology (2016) 26: 1404-15. *Corresponding author
Luo, C.J., Wightman, R., Meyerowitz, E.M., and Smoukov, S. 3D fibre scaffold as an investigative tool for studying the morphogenesis of isolated plant cells. BMC Plant Biology (2015) 15: 211.
Peaucelle, A., Wightman, R., Hofte, H. The control of growth symmetry breaking in the Arabidopsis hypocotyl. Current Biology (2015) 25: 1746-1752.

