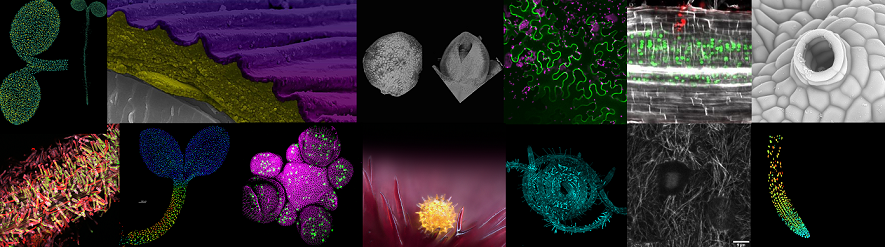
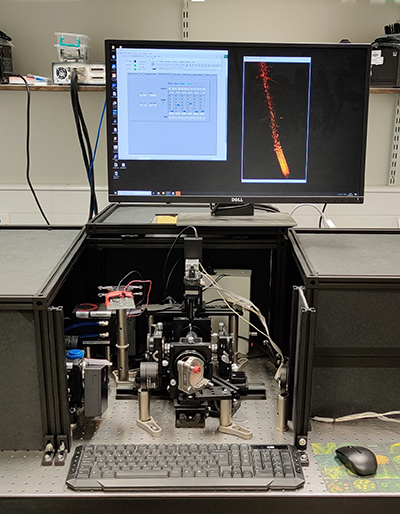
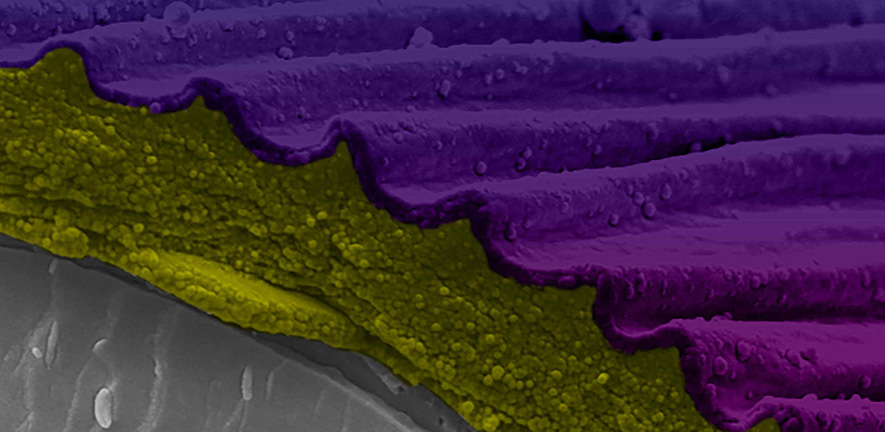
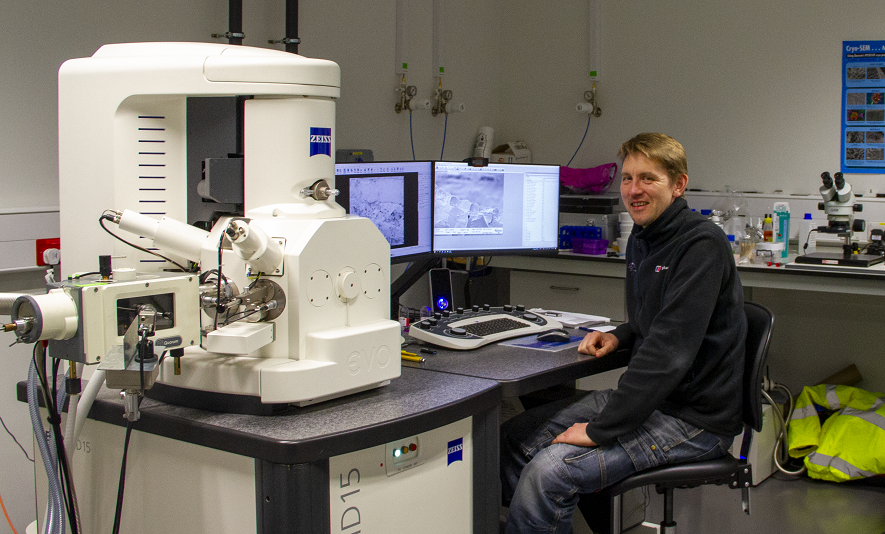
Sainsbury Laboratory Microscopy Core Facility
The Sainsbury Laboratory Microscopy Core Facility provides state-of-the-art advanced imaging and analytical tools for plant developmental biology research at the Sainsbury Laboratory and departments across the University of Cambridge.
We are the largest core microscopy facility at the University of Cambridge with a suite of 23 individual microscopes. We provide live imaging of developing plant tissues, widefield microscopy, atomic force microscopy, high spec colour bright field imaging, raman imaging and high-resolution cryo-scanning electron microscopy.
More than 12,000 hours are logged on our microscopes each year supporting more than 80 research projects. The facility is funded and maintained by the Gatsby Charitable Foundation.
The team
Dr Raymond Wightman
Imaging Core Facility Manager
+44 (0)1223 761139
raymond.wightman@slcu.cam.ac.uk
Gareth Evans
Microscopy Specialist
gareth.evans@slcu.cam.ac.uk
Dr Martin O. Lenz
Imaging Research Associate
mol21@cam.ac.uk
Imaging techniques and resources
The facility provides a wide portfolio of imaging platforms covering a number of imaging techniques using confocal and fluorescence microscopes, digital imaging, light-sheet, cryo-scanning electron microscopy (cryo-SEM), atomic force microscopy and chemical imaging (raman). Our microscopy core facility supports a diversity of plant science research, including cell and tissue mechanobiology, plant developmental biology, phytohormones, molecular signalling and plant-microbe interactions.
We work alongside computer scientists to develop a range of tools applied to live imaging in the plant sciences. This encompasses 3D visualisation, cell segmentation and quantification from large tissue datasets, and lineage analysis of large 4D datasets, which is then fed in to computer models.
A new light sheet system designed for rapid fluorescence imaging of whole roots, seedlings and plant organs was built by and is maintained by Dr Martin Lenz as part of a collaboration between the Sainsbury Laboratory and Cambridge Advanced Imaging Centre.
Innovations in microscopy
Advanced light-sheet microscopy for plant science research
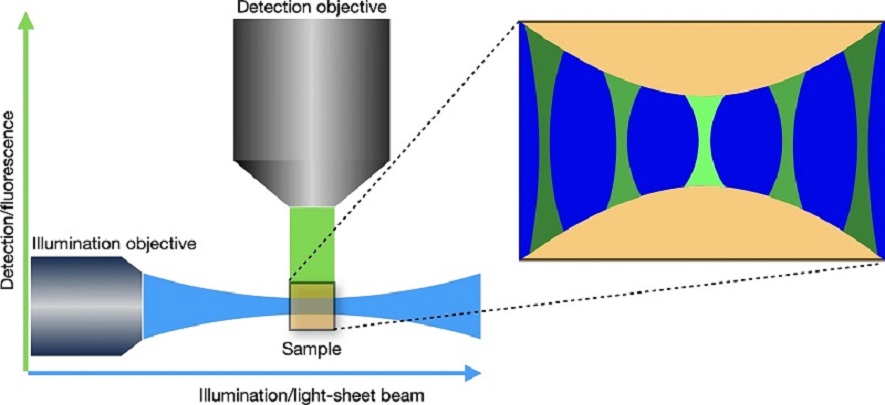
A custom-built scanning-light-sheet microscope has been designed for rapid vertical imaging of whole roots, seedlings and plant organs. It is further optimised for whole-plant FRET analysis. The light sheet system also incorporates equipment for mechanical manipulations. We collaborated with the Cambridge Advanced Imaging Centre to build this dual-scanning-light-sheet microscope specifically tailored to plants. Both live and cleared samples are imaged vertically in a bespoke imaging chamber containing two illumination objectives and two imaging objectives. The system allows imaging of both sides of a sample concurrently and a rotating sample holder permits multi-angle views of the sample.
Customised microfluidics
A new bespoke microfluidics platform comprises a Kloe Dilase 650 laserwriter with associated equipment for cleanroom-level manufacturing of fluidic devices is another innovation tool supporting life sciences research. The 375nm laser accurately constructs channel arrays with nanometre precision on silicon wafers that are then used as templates for holding bacteria and single plant cells during live cell imaging.
Chemical imaging
Finally, chemical imaging techniques have been developed (and published) using the Leica-Renishaw SP8-“FLIMan” prototype.
Data analysis
One of the core strengths of our facility is that we work alongside computer scientists at the Sainsbury Laboratory to develop a range of tools applied to live imaging in the plant sciences: 3D visualisation, cell segmentation and quantification from large tissue datasets, lineage analysis of large 4D datasets. This data is then fed in to computer models that describe the dynamics of gene regulatory networks, hormone transport and signalling, cell growth and division, and mechanical properties.
Novel biosensors and biomechanics tools
Sainsbury Laboratory research teams are also developing new tools to visualise the dynamics of plant systems. The research team of Alexander Jones is developing biosensors for confocal microscopes that enable plant scientists to track in real time the molecules behind plant growth and Sarah Robinson's research group is devising novel biophysical tools such as the ACME robotic system to measure the mechanical properties of plants.
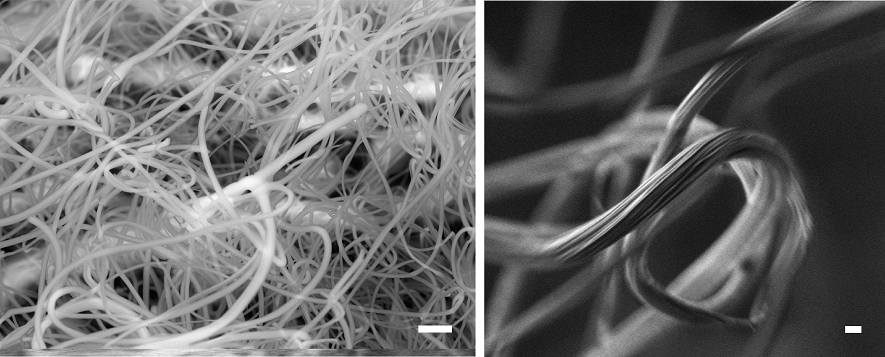
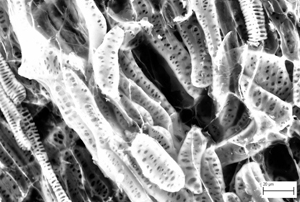
Scanning electron microscope images of woolly fibres produced on the leaves of the alpine plant Dionysia tapetodes. Images by Raymond Wightman and Trevor Groves.
Spotlight on research outcomes
Our microscopy facility has supported a number of high impact discoveries in areas such as:
- Complex cell morphology (molecular mechanism of cytokinin-activated cell division in Arabidopsis and Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells)
- 4D cell lineage tracking during flower development
- Wood ultrastructure
- Petal biomechanics
Find out about other plant science research discoveries made at the Sainsbury Laboratory Microscopy Core Facility:
- Putting plants under the microscope
- Rare mineral discovered in plants for first time
- Alpine plant spins its own flavonoid wool
- Plastic-free mountaineering clothing inspired by alpine plants unveiled at Milan Design Week
Types of microscopes
Microscopes include:
- Light Microscopy
- Macro and stereo-imaging
- 3D stereo, digital and phase-contrast microscopy
- Epifluorescence microscopy
- Differential interference contrast (DIC) microscopy
- Confocal microscopy
- Light sheet imaging
- Scanning Electron Microscopy
- Atomic Force Microscopy
Information about each microscope is provided below:
Stereo microscopy, macro-imaging and digital photography
Dissecting and stereo microscopes
We have transmitted light stereo microscopes for imaging small samples, including whole-mounts, culture plates, gels and tissue sections. Equipment includes:
- 15 Zeiss Stemi dissecting/stereo microscopes distributed across the main building and building B
- 2 Olympus SZ61 stereoscopes for tissue culture work
- 1 Olympus stereoscope fitted with an SLR camera for taking images/movies during dissection
Olympus stereoscope with SLR
This is a portable stereo-microscope custom built for the Sainsbury Laboratory to take advantage of the features of the attached Nikon SLR camera. It is particularly good for taking photos and recording movies during routine dissection. A ring LED light provides even illumination of the sample below and further modifications have increased the workable space below the optics allowing imaging/dissection of plants whilst still in their pots. The “Comfort View” eyepieces reduces user fatigue. Zoom up to 45x.
Digital photography
A dedicated photography studio is located in Building B (Chamber 10) for taking photos of larger items, for example plant trait phenotyping of mature plants or seedling trays. The studio has lights, a Nikon D5200 SLR with standard and zoom lenses, black backdrop fabric and tables.
3D stereo and digital microscopy
Leica M165C
The Leica M165C designed for imaging and plate/slide screening and can take images using the attached SPOT Flex colour and monochrome camera with PC using the SPOT software. It contains a wide range of filter options for UV, CFP, GFP, YFP and Red fluorescent proteins. It also has a matched CFP-YFP FRET filter and a wide-emission GFP filter for viewing the green GFP signal over the red chlorophyll autofluorescence. Light can be provided from above either through goosenecks or a ring light.
Leica Thunder Imager Model Organism
The Leica Thunder Imager Model Organism is a powerful stereofluorescence microscope designed for imaging and plate/slide screening. Images can be taken on either a Leica K3 colour camera or a powerful Leica K8 monochrome camera using Leica LASX software. The Navigator module allows whole plates to be scanned quickly and then regions of interest selected for higher resolution scans. The Thunder module provides advance computational clearing to improve the signal to noise ratio of images taken. It contains a wide range of filter options for UV, CFP, GFP, YFP and Red fluorescent proteins. It also has a matched CFP-YFP FRET filter and a wide-emission GFP filter for viewing the green GFP signal over the red chlorophyll autofluorescence. Fluorescent light is provided above from a Leica LED3 and brightfield light is from below. Objectives include a 1x and a 5x.
Zeiss V20 Stereo
The Zeiss V20 stereo has various objectives and multiple lighting modes. The V20 is particularly good for amalgamating z-stacks into a single in focus extended-depth-of-field image.
The V20 is for generating publication quality images and offers various methods of sample illumination; from above using the fully programmable ring light or from below. It is attached to a high resolution colour camera and is operated through the PC using Zen Blue software. A key function of the V20 is the extended-depth-of-field acquisition where all out of focus data is removed from a large z-stack giving a single in-focus image.
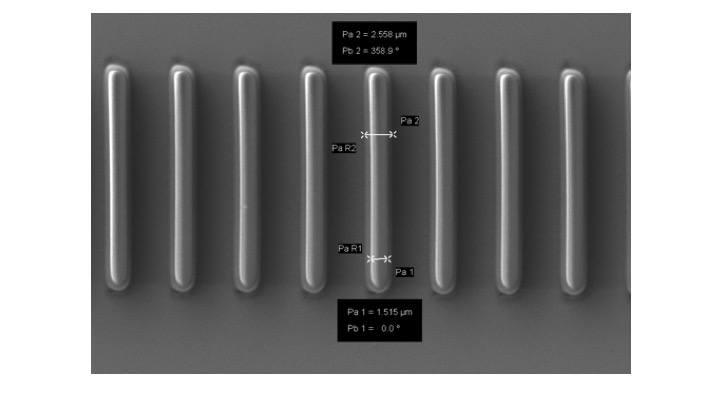
Keyence VHX-5000 digital microscope
The Keyence VHX-5000 allows any surface to be viewed in-focus with its Real Time Depth Composition. Ideal for any surface from flat to uneven. Switching from low to high magnifications is easy with the range of zoom lenses from 20x to 1000x.
Features:
- 3D display of surfaces
- 50 frames/sec CMOS image sensor
- High resolution HDR for picking out detailed colour gradients
- 2D and 3D measurement tools.
Keyence VHX-7000 digital microscope
The Keyence VHX 7000 allows for rapid wide-area scanning of all surfaces to give high resolution in-focus images at 4K ultra-HD resolution. The integrated head allows for low to high mag imaging without manual change of objectives. Improved lighting modes with less glare permits imaging of shiny and reflective specimens.
Features:
- Magnification range 20x to 6000x with automated selection of objectives via the motorized turret
- 3D measurements
Keyence VHX-X1 digital microscope
The Keyence VHX X1 allows for rapid wide-area scanning of all surfaces to give high resolution in-focus images at 4K ultra-HD resolution. The integrated head allows for low to high mag imaging without manual change of objectives. Improved lighting modes with less glare permits imaging of shiny and reflective specimens.
Features:
- Magnification range 20x to 2000x with automated selection of objectives via the motorized turret
- 3D measurements
- Search light mode allows for optimum lighting selection depending on sample
Leica DM1000 phase-contrast microscope
The Leica DM1000-wifi is a portable microscope offering standard brightfield or phase-contrast illumination with Kohler illumination that is simple to set up. Within the camera body is a 5MP colour wifi camera that streams images to your phone or tablet using the freely available Leica AirLab app.
The app enables a variety of controls such as exposure, white balance, scale and other annotations. Battery packs enable the microscope to be used without an external power source.
Features:
- Phase-contrast optics
- Leica ICC50 W camera with wifi (requires free iOS/Android app), HDMI and USB outputs
- SD card port
- Stain-free, easy-clean stage
- Objectives: 4x, 10x, 20x, 40x, 100x oil immersion.
Epifluorescence microscopy
In epifluorescence microscopy (also called widefield microscopy), a parallel beam of light is passed directly upwards through the sample, maximizing the amount of illumination.
The excitation and emission light travel through the same objective lens in epifluorescence microscopy, where “epi” is borrowed from the Greek to mean “same”. Epifluorescence microscopy is particularly useful for imaging thick samples.
Life Technologies EVOS FL
Life Technologies EVOS FL is particularly good for total beginners to microscopy. It uses light cubes that cover a wide range of applications. It provides quick, high quality images.
There are no eyepieces, instead an easy-to-use interface enables the user to take quick snapshots through to time-lapse imaging using the in-built monochrome camera. Fluorescence illumination is provided by LEDs within specific light cubes for imaging a variety of fluorescent proteins and dyes.
- Light cubes available for: DAPI, CFP, YFP, FRET, GFP, RFP, TexasRed, Brightfield
- Objectives: 1.25x, 4x, 10x, 20x, 40x and 100x (oil immersion)
Inverted Fluorescence Microscope (Leica DM IL LED)
This inverted fluorescence microscope is best used for screening.
- Filters: DAPI, GFP, RFP and brightfield
- Objectives: 5x, 10x, 20x, 40x (all dry)
- Phase contrast (with 40x objective only)
Differential interference contrast (DIC) microscopy
Differential Interference Contrast (DIC) imaging is a technique used to increase contrast in brightfield images.
DIC microscopy uses polarized light to illuminate the specimen and as a result images have significantly better resolution and higher contrast even at low magnifications and long working distances. Laser scanning DIC images can be acquired simultaneously with fluorescence images and provide useful morphological information.
Zeiss Axioimager M2
The Zeiss Axioimager M2 is a wide field epi-fluorescence/transmitted light upright microscope that is capable of performing epi-fluorescence imaging in combination with transmitted-light brightfield, darkfield and DIC.
It is fitted with 3 cameras: 16-64 megapixel colour and standard colour for brightfield plus a sensitive Hamamatsu 1k x 1k EMCCD for fluorescence. The EMCCD, when used in conjunction with the Zeiss fast acquisition module, is capable of speeds in excess of 50 frames per second with exposure times as low as milliseconds for weak signals and microseconds for strong signals.
Software
- Axiovision incorporating modules Inside4D, High Dynamic Range, Fast Acquisition and Physiology.
- Axioimager filters for fluorescence imaging: DAPI, FITC/GFP, DsRED, YFP, CFP, FRET
Objectives
- PlanApochromat 10x /0.3
- PlanApochromat 20x/ 0.8
- EC Plan-NeuFluar 40x/ 0.75
- PlanApochromat 63x/ 1.4 (oil immersion)
- EC Plan-NeuFluar 100x/ 1.3 and (oil immersion)
- 60x/1.2 water immersion objective can be fitted upon request
Confocal microscopy
We have 6 point scanning confocal microscopes.
Zeiss LSM880
The LSM880 upright confocal is particularly particularly good for imaging problematic fluorophore combinations and achieving better resolution than conventional confocal microscopes. It is suitable for high quality 3D imaging using combinations of fluorescent proteins or dyes that would otherwise be difficult to distinguish. It can fit larger objects via the attached Axio-Examiner microscope stand, making it particularly useful for time-lapse imaging of whole plants. The Airyscan module permits super-resolution imaging, achieving lateral resolutions of up to 120nm.
Software
Zen confocal software including rapid 3D rendering
Lasers
405nm, 440nm, 458nm, 488nm, 514nm, 561nm, 633nm
Detectors
- 2xPMTs, 1x Spectral detector (32 Channel GaAsP QUASAR detector)
- 1x Airyscan detector
- T-PMT
Extras
Motorized stage
Objectives
- Dry: 10x, 20x
- Immersion: 40x 1.3NA oil, 63x 1.2NA water, 63x 1.4NA oil
- Water dipping: 10x, 20x, 40x, 63x 1.0NA
Leica Stellaris 8
Upright confocal equipped with sensitive HyDs detectors. A white light laser allows freely tuneable excitation from 440nm to 790nm. TauTools enables fluorescence signal to be separate based on average lifetime information and gating can remove unwanted autofluorescence. This confocal has a vertical imaging mode, enabling roots grown vertically on petri dishes to be easily imaged as part of a time-lapse experiment. It is fully automated and particularly suitable for fast image acquisition. It contains both the tandem and resonant scanner for routine and high speed imaging, respectively. All detectors are high sensitivity HyDs detectors suitable for low-light imaging.
The SP8 can be converted to a vertical imager in 30 minutes, enabling seedlings to be imaged directly on specific petri dishes as they grow and respond normally to gravity. This involves the microscope nosepiece illuminating to the side. A high performance piezo focusing kit enables rapid z-stacks to be taken and Live Data Software Mode enables multiple regions to be visited.
Software
Leica LASX with Matrixscanner module and FRAP tools and TauTools
Lasers
405nm, White light laser 440nm - 790nm
Detectors
3x HyDs, 1x T-PMT
Objectives
- Dry: 5x, 10x, 20x
- Immersion: 63x 1.2NA water, 63x 1.4NA oil, 100x 1.44NA oil, 20x 0.75NA multi-immersion for long working distance/bleaching
- Water dipping: 25x 0.95NA, 40x 0.8NA, SP8-Vertical Imager mode
Extras
Piezo z-drive
Zeiss LSM700
The LSM700 upright confocal microscope is particularly good for combinations of GFP/YFP with the new generation of fluorescent proteins (e.g. mOrange/mCherry). It is fitted with custom filter sets that reduce the amount of undesirable autofluorescence from live plant samples. A new automatic stage, with the same controls and "feel" of a manual, facilitates easy location of samples/regions of interest while enabling multi-position visiting and tile-scanning.
Software
Zen confocal software including rapid 3D rendering.
Lasers*
405nm, 488nm, 555nm, 639nm
*all solid state
Detectors
2xPMTs, T-PMT
Objectives
- Dry: 10x, 20x
- Immersion: 40x 1.3NA oil, 63x 1.2NA water, 63x 1.4NA oil
- Water dipping: 10x, 20x, 40x, 63x 1.0NA
Leica-Renishaw SP8-FLIMan
This system is a new type of confocal enabling label-free organelle imaging and Raman detection of molecules. It enables you to image, in living tissue, the ‘chemistry of cells’. A partnership between the Sainsbury Laboratory and three companies; Leica Microsystems, Leica Industrial and Renishaw, the FLIMan allows a diverse range of specialist techniques in addition to high-end confocal microscopy:
- Real time imaging of protein-protein interactions and biosensors using FLIM
- Label-free organelle imaging using Raman, in addition to fluorescently labelled reporters
- Direct imaging of molecular constituents of cells and tissues with applications to phytohormones
Leica SP8 confocal with dual channel FLIM, 2x HyD SMD detectors, 2x PMTs, 1x t-PMT, automatic stage for use in both confocal and Raman modes. Renishaw InVia Raman system with Rencam CCD and Andor EMCCD detectors. Conversion kit for liquid samples.
Software
Leica LAS X with FLIM module, Picoquant software for photon counting. WiRE software for Raman spectral acquisition and
mapping.
Lasers
(Confocal) Pulsed 405nm, 440nm, 470nm. Continuous 440nm, 488nm, 514nm and 552nm
(Raman) 532nm, 785nm.
Objectives
(SP8+Raman) 20x dry, 20x 0.75NA multi-immersion, 25x 0.95NA water dipping, 63x 1.2NA water, 63x 1.4NA oil.
(Raman only) 5x dry, 20x dry, 50x dry, 20x 0.75NA multi-immersion.
Leica SP8-iPhox
The Leica SP8-iPhox inverted confocal is equipped with cooled ultra-sensitive detectors and all-solid-state lasers. This confocal has been designed for high-end applications, including automated time-lapse imaging over long periods, single photon detection, rapid scanning and detailed quantification.
The Leica SP8-iPhox uses solid state lasers and ultrasensitive detectors for rapid low-signal imaging. Hardware autofocus prevents unwanted drift during time-courses and a new Stage Overview mode allows quick and easy scanning over large areas.
Equipment
Leica SP8 on DMI8 inverted microscope. 2x HyD SMD detectors, 2x PMTs, 1x t-PMT, fully automated stage, hardware autofocus, Resonant scanner option for high-speed scanning.
Software
LAS X with Live Data mode
Lasers
Solid-state 405nm, 448nm, 488nm, 514nm, 552nm.
Objectives
- Dry: 5x, 10x, 20x
- Immersion: 63x 1.2NA water, 63x 1.4NA oil
- Upon request: 100x 1.44NA oil and 20x 0.75NA multi-immersion
Leica StretchP8
The Leica StretchP8 upright confocal contains solid state lasers for imaging and time-lapse of whole plants, organs and tissues, together with mechanical perturbations using external devices mounted on the stage. The confocal supports single cell ablations and has the Lightning module that allows on-the-fly image processing to extract more information during scanning to give finer detail.
Equipment
Leica SP8 on upright microscope. 2x HyD detectors, 1x PMT, 1x t-PMT, automated xy stage
Software
LAS X with Lightning module
Lasers
Solid-state 405nm, 488nm, 552nm
Objectives
5x, 20x dry, 20x 0.5NA, 25x 0.95NA and 63x 0.9NA water dipping
Light sheet imaging
Custom-built light sheet microscope for vertical imaging of whole plants
Our light sheet system has been designed for rapid fluorescence imaging of whole roots, seedlings and plant organs. It was built and is maintained by Martin Lenz as part of a collaboration between the Cambridge Advanced Imaging Centre and Sainsbury Laboratory.
Both live and cleared samples are imaged vertically in a bespoke imaging chamber containing two illumination objectives (for generating the light sheets on both sides of the sample) and two imaging objectives. The system allows imaging of both sides of a sample concurrently and a rotating sample holder permits multi-angle views of the sample. The system supports all the major fluorophores plus FRET-based biosensors. One imaging objective can be combined with a magnification changer to increase magnification.
Lasers
405nm, 445nm, 488nm, 515nm, 561nm, 638nm
Detection
2x back illuminated sCMOS camera (Hamamatsu Orca Flash4)
Objectives
Within the sample chamber are two illumination objectives and two imaging objectives:
- Illumination: 2x Nikon 10x, 0.3NA, water dipping
- Imaging: 2x Olympus 20x, 1.0NA, water dipping
Scanning electron microscopy
We have two scanning electron microscopes - Zeiss EVO HD15 and Hitachi TM4000PLUS Desktop SEM.
Zeiss EVO HD15 Scanning Electron Microscope
Both conventional and cryo Scanning Electron Microscopy is carried on a Zeiss EVO HD15 that is equipped with various detectors. Preserved or dried material can be first coated with Gold using the Safematic CCU-010 and imaged in high vacuum. Fresh (hydrated) material can be rapidly frozen in nitrogen slush, cryo fractured and coated with either Gold/Palladium, Platinum or Iridium and imaged on the cryo-stage in the SEM chamber.
The EVO HD cryoSEM, built by Zeiss and Quorum technologies, allows high magnification imaging of cryo-preserved tissue in full vacuum conditions. Magnifications routinely achieved are between 90x and 50,000x with maximum magnification of approximately 300,000x. Excellent contrast is maintained through the use of a built-in gold/palladium, platinum, iridium sputter coater enabling user-defined coating on the nanometre scale. Fracture tools enable fractures through organs and cells revealing delicate structures in the cell walls and membranes. Sublimation protocols enable removal of surface ice or reduction of cytoplasm to reveal organelles. The time between plunge-freezing and imaging takes as little as 15 minutes.
Specifications
Filament: LaB6
Detectors: SE, VPSE G3, EPSE, BSD (5 segment), STEM (for single TEM grids)
Stage: (standard SEM mode) 9 place stage or single specimen Debien Coolstage. For cryoSEM a cryo stage maintains samples at –145C with an anti contaminator maintained at –175C.
Cryo prep-deck: maintained at –145C with two fracture knives, platinum sputter coater with thickness monitor. Associated workstation for plunge freezing of fresh tissue with cryo-transfer under vacuum.
Utilising a Zeiss V12 Sterofluorescence microscope the team are working on a protocol for cryo-fluorescence microscopy to use fluorescence to guide high mag SEM imaging
Software
SmartSEM including SmartStitch module for tile imaging of large areas at high mag.
Access and technical support
cryoSEM drop-in sessions, where users bring samples for cryo preparation and imaging by the facility manager, run from Wednesday 1pm to Friday evening. Please contact the facility manager to reserve a place.
Associated equipment consists of a Safematic Gold sputter coater that can apply nanometre-precision coatings.
Hitachi TM4000PLUS Desktop SEM
The Hitachi TM4000PLUS Desktop SEM is equipped with a Deben CoolStage and has a low vacuum mode for quick SEM imaging of fresh plant material without coating.
It has Backscatter (for routine images of cell outlines) and SE detectors (for high resolution) and can switch between low and high vacuum modes. The chamber takes approximately 2 mins to pump to vacuum allowing for quick imaging of multiple samples. The CoolStage operates between -50C - +50C. The 4 segment backscatter detector combined with Mountains Map software enables 3D reconstructions of images to be generated.
Semplor Nanos Desktop SEM
Similar to the Hitachi, the Semplor Nano Desktop SEM has a low vacuum mode which is lower than the Hitachi meaning it is gentler of fresh biological specimens negating the need for a cold stage. Images can be taken using a backscatter detector in low vac mode and an SE detector in high vac mode. The large chamber and sample holder without a cold stage mean that much large specimens can be imaged in this desktop SEM.
Atomic force microscopy
We have a Bruker Biowizard 5 AFM, manufactured by JPK Instruments. Housed in an acoustic enclosure it permits sensitive mechanical measurements of biological samples down to the subcellular level. It features a BioMat workstation for correlative light-AFM imaging. The attached Zeiss Axio Zoom V16 allows for fluorescently label samples and regions to be imaged at high precision by the AFM and force measurements taken from those specific areas.
Photolithography Facility
The photolithography facility contains various equipment for making microfluidic devices based on custom designs. These devices can then be used for the study and manipulation of bacteria, single isolated plant cells, whole tissues and seedlings. They are often used for long term imaging experiments on the various SLCU microscopes.
Kloe Dilase 650 LaserWriter
The Dilase 650 is a high-resolution direct laser lithography system. It has a 375nm laser which can be focused on either a 1mm or 10mm spot sizes. The system allows the writing on any type of substrate (photomask, semiconductors, glass, polymers or crystals) over a surface area of up to 4 in2.
The Dilase 650 is designed for etching out both simple and complex patterns of channels and cavities for custom building of microfluidic devices. Its 375nm laser accurately constructs channel arrays with nanometre precision on silicon wafers that are processed in a cleanroom environment and then used as a template, or mould, for accommodating bacteria and single plant cells during live cell imaging.
UV Lamp
365nm UV lamp. This can be used for exposing larger features with a photomask.
SUSS LabSpin 6 Spin Coater
Suss spin coater for the coating of thin layers (less than 10mm) of photoresist onto wafers. A range of chucks are available to accommodate different wafer sizes and shapes.
Polis Spin Coater
Polis spin coater for the coating of thicker layers (greater than 10mm) of photoresist.
Stuart Hotplates
3x Stuart Hotplates with a temperature range up to 350°C for curing the photoresist before and after exposure.
Grant-bio Orbital Shaker
Grant-bio orbital shaker for the agitation of wafer cleaning solutions prior to spin coating and the development of wafers post exposure.
Leica DM2700 M Microscope and Camera
Leica DM2700 M microscope and HD camera for checking microfluidic features after development. Objectives include 5x, 20x, 50x, 100x and 150x.
For access to the facility please contact Gareth Evans or Christian Schwall.
Resources and support facilities
Microscopy support facilities include a well-equipped preparation room, sample incubation growth chamber, uninterrupted back-up power supply (for high-end confocal and SEM systems, backed-up data storage on a central and remote server and image analysis workstations.
- Histology equipment
- Preparation room
- Laser writer for microfabrication of microfluidics devices
- Growth chambers
- Image analysis software
Histology
We have versatile histology equipment to preserve and visualise micro-anatomy of model organisms, including:
- Sample collection, fixation and processing
- Cryo- and paraffin embedding and sectioning
- Staining
Microtome (in situ)
Microstomes
Plate Scanner
Pelco Biowave
Preparation Room
The microscopy preparation room contains specialised equipment and consumables including:
- Dissecting microscopes
- Pipettes, forceps, tips, sterile water
- Slides, coverslips, dished and boxes for specimen preparation
- Waterbath and microwave
- Fridge and freezer for temporary storage of samples and reagants
- A selection of dyes and imaging reagants from Life Technologies
Growth chambers
Grobotic Systems growth chambers
For incubation of plant samples during microscopy there is a Grobotic Systems growth chamber situated close to the microscopes. This chamber is set to 22°C, constant, 150mmol light. The interior environment of the chamber is monitored continuously and minute-by-minute logging of the interior environment allows users to troubleshoot any problems during their experiment.
A second Grobotic Systems growth chamber is also available. This can be used as an over-spill during busy periods for the other chamber but is also bookable for individual experiments. Temperature, light duration and intensity can be independently set for day/night cycles. An internal camera can be used for timelapse movies for plant phenotyping and plant growth.
Image analysis
The facility has 3 dedicated powerful image analysis workstations with software including Imaris (4D rendering an tracking), Huygens (deconvolution and 3D rendering), Morphographics (segmentation and tracking) and FIJI as well as offline versions of the software used to run the facility microscopes.
Expertise and training
Our team's expertise covers a range of imaging and analytical tools developed for the plant sciences. The Microscopy Facility team has many years of experience light, fluorescence and electron microscopy and actively support researchers with setting up a new study, troubleshooting or helping to sort out an unexpected result. They provide inductions and training on all microscopy equipment and specialist techniques.
Access and booking
In addition to members of the Sainsbury Laboratory, we also also support research carried out in other University of Cambridge departments including Plant Sciences, Biochemistry, Neuroscience, Materials Science and Geology. You must complete an induction and training for the specific microscope before you will be able to book it.
Contact
Dr Raymond Wightman
Imaging Core Facility Manager
+44 (0)1223 761139
raymond.wightman@slcu.cam.ac.uk